Abstract
The purpose of this study was to evaluate the safety, tolerability, pharmacokinetics, and possible antitumor activity of a ligand fusion-protein, DAB389IL-2, in a phase I trial. This was a multicenter, open-label, dose-escalation trial. Patients with preserved organ function and histologically confirmed relapsed cutaneous T-cell lymphoma (CTCL), other non-Hodgkin's lymphomas (NHL), or Hodgkin's disease (HD) were eligible if their cancer was shown to express the interleukin (IL)-2 receptor by an immunohistochemical assay for the p55 or the p75 subunit. Patients received up to eight courses of DAB389IL-2 given as a short intravenous infusion daily for 5 days with subsequent courses every 21 days. The maximum tolerated dose (MTD) and tumor response was determined according to standard criteria. Seventy-three patients (44 men/29 women), aged 16 to 81 years (mean, 50.7) with CTCL (n = 35), NHL (n = 17), and HD (n = 21) were enrolled. The patients were extensively treated, failing 0 to 15 previous therapies (median, 4). Patients received one to six courses (mean, 3.3) of DAB389IL-2 over a range of 3 to 31 μg/kg/day. The dose-limiting toxicity was asthenia, establishing the maximum tolerated dose of 27 μg/kg/day. Approximately half of all patients had significant titers of antibody to diphtheria toxin or to DAB389IL-2 at the time of enrollment compared with 92% with titers at the end of treatment. The presence of antibody did not preclude clinical response. There were five complete (CR) and eight partial (PR) remissions in patients with CTCL with one CR and two PR occurring in NHL. The median time to response was 2 months and the duration of response was 2 to 39+ months. No responses were documented in patients with HD. DAB389IL-2 is well tolerated with an MTD of 27 μg/kg/day. This ligand fusion-protein showed antitumor effects in patients with IL-2 receptor expressing CTCL and NHL. Additional trials in these diseases are warranted.
DAB389IL-2 IS AN INTERLEUKIN-2 receptor (IL-2R) specific ligand fusion-protein produced by expression of a recombinant fusion gene in Escherichia coli. This fusion gene consists of nucleotide sequences of the enzymatically active fragment A of diphtheria toxin (DT), the membrane-translocating portion of DT fragment B and human interleukin-2 (IL-2). DAB389IL-2 binds specifically to high-affinity IL-2R in vitro and is rapidly internalized via receptor mediated endocytosis. Once internalized into an acidic vesicle, the enzymatically active fragment A portion is released into the cytosol. Protein synthesis is inhibited via fragment A-catalyzed adenine diphosphate (ADP) ribosylation of elongation factor 2 and ultimately results in cell death.1 The potential for DAB389IL-2 to be selective for IL-2 receptor expressing malignancies in conjunction with its novel mechanism of action provide the rationale for the development of this agent in humans.
An earlier version of this ligand fusion-protein, DAB486IL-2, was shown to be well-tolerated and to demonstrate antitumor activity in patients with advanced hematologic malignancies.2-7 DAB389IL-2 differs from the earlier DAB486IL-2 in the deletion of 97 amino acids from a portion of the native receptor binding domain. The resulting protein is of lower molecular weight (57 kD v 68 kD) than DAB486IL-2. This modification results in a fivefold improvement in affinity of DAB389IL-2 for the IL-2R and approximately 10-fold increase in potency. The newer ligand fusion-protein also has an extended half-life compared with the earlier version.
The purpose of this study was to evaluate the safety, tolerability, pharmacokinetics, and possible antitumor effects of DAB389IL-2 in patients with recurrent, IL-2R expressing cutaneous T-cell lymphoma (CTCL), Hodgkin's disease (HD), and non-Hodgkin's lymphoma (NHL).
MATERIALS AND METHODS
Description of DAB389IL-2.
DAB389IL-2 is purified from inclusion bodies by reverse-phase chromatography and a series of diafiltration steps. The final product has a molecular weight of 58 kD. DAB389IL-2 was supplied as a frozen injectable solution formulated in 2-mL single-use vials at a concentration of 150 μg/mL. Six lots of drug were used in this study. The last two lots of drug were formulated in a citrate buffer instead of the phosphate buffer used previously. This change was made to further improve the stability of DAB389IL-2.
Protocol design.
The protocol was conducted under a United States Investigational New Drug Application, and together with the informed consent was approved by the institutional review board of each institution. Written informed consent was obtained from each patient before enrollment.
Patients of legal consenting age of either sex were eligible for treatment if they had CTCL, other NHL, or HD disease. Pathologic confirmation and determination of IL-2R expression on malignant cells was required within 6 months of enrollment or after most recent therapy. Patients were required to have an evaluable tumor burden, a performance status of >70% (Karnofsky), and preserved organ function.
Patients were enrolled in cohorts of three to successively increasing dose levels of DAB389IL-2 beginning at 3 μg/kg/day. Subsequent dose escalation in an individual patient was not allowed. If one patient had a dose-limiting adverse experience at a given level, at least three and as many as six patients expanded the level. The maximal tolerated dose (MTD) was defined as the level at which two of six patients experienced an agent-related dose-limiting (grade III or IV) adverse event. Toxicities were scored according to the Common Toxicity Criteria established by the National Cancer Institute for phase I trials.
A treatment cycle consisted of DAB389IL-2 administered as a short infusion over 5 minutes daily for 5 days. Treatment cycles were repeated every 3 to 4 weeks. Patients experiencing infusion-related fever and chills had the infusion prolonged to 15 minutes and received acetaminophen and diphenhydramine as premedication. Patients experiencing rigors received intravenous meperidine for symptomatic relief.
Patient evaluation.
Clinical and laboratory parameters were followed closely for toxicity. Laboratory studies including serum chemistries and tests of liver function were performed on day 1 of treatment and twice weekly for 3 weeks. Routine blood counts were performed weekly. Treatment-related laboratory or clinical abnormalities must have resolved before initiation of the next cycle. Patients were allowed to postpone retreatment for 1 week to allow resolution of toxicities.
Immunohistochemistry.
Expression of either the p55 or p75 subunit of the IL-2R by malignant cells was a requirement for enrollment. Frozen tissue specimens were submitted to a central pathology laboratory. The diagnosis was confirmed by examination of hematoxylin and eosin stained frozen sections, paraffin sections (where necessary) and the immunophenotype. Additional studies were conducted as needed to properly characterize the immunophenotype of the malignancy.
Immunohistochemistry was performed on frozen sections with the following antibodies: CTCL-CD2, CD3, CD4, CD8; NHL-CD22, kappa, lambda; HD-CD30, CD15, CD3, and CD22. In addition, the samples were analyzed for the alpha (p55) (CD25, Dako, Carpenteria, CA, clone ACT-1) and beta (p75) (Accurate Chemical and Scientific Corp, Westbury, NM, clone Mik B.) subunits of the IL-2R using a standard three-step labeled streptavidin biotin method with diaminobenzidine chromagen and osmium intensification (Dako).8 The staining proportion was estimated. Cases where the tumor cells stained for either the alpha or beta component of the receptor were considered positive and therefore eligible for the study.
Tumor expression of the IL-2R was determined on the basis of the following criteria: (1) Presence of malignant cells in the specimen was determined on the basis of histologic assessment and immunophenotypic characterization. (2) Detection of IL-2R was based on staining with anti–IL-2R antibodies on the cells in the serial section. The expression was graded as strong or weak, and the proportion of malignant cells expressing the IL-2R was determined. (3) Samples with high background staining were scored indeterminate. (4) Samples with IL-2R expression by reactive infiltrating lymphocytes were considered to be undetectable or indeterminate depending on the factors that could be used to discriminate between the malignant cells and the infiltrating lymphocytes. For example, in a B-cell lymphoma specimen, a pattern of staining for IL-2R that was similar to the pattern for B-cell markers would be evidence of IL-2R expression by the malignant cells, whereas a pattern of staining similar to the pattern for T-cell markers would be suggestive of IL-2R expression by infiltrating reactive T lymphocytes. IL-2R expression on Hodgkin's cells was determined by identifying uniform cytoplasmic and membrane immunohistochemical staining on large, cytologically abnormal Hodgkin's variant cells. Many of the adjacent T lymphocytes were positive for the receptor. Therefore, care was taken to not misinterpret staining of reactive cells. (5) Expression of the alpha subunit of the IL-2R (p55) was assessed separately from the beta subunit (p75).
Pharmacology.
On stipulated days of dose administration, serum samples were obtained for pharmacokinetic analysis. Three of the clinical sites (South Texas Cancer Institute, University of Alabama at Birmingham, and Northwestern University) were to obtain full pharmacokinetic sampling; the rest only obtained two samples for partial pharmacokinetics. Samples were to be drawn on days 1 and 5 of course 1, and by amendment later in the study, for course 3. Samples were obtained predose, end of dose, 5, 10, 15, 30, 45, 60, 120, 180, and 240 minutes postdose and immediately frozen. Samples were assayed for serum levels as bioactive (bioassay) and immunoreactive (enzyme-linked immunosorbent assay [ELISA]) DAB389IL-2. The product ELISA is based on the ability of two different monoclonal antibodies to detect immunoreactive DAB389IL-2.
Antibody and soluble IL-2R assay.
Patients were screened for soluable IL-2R and for antibodies to DAB389IL-2, DT, and IL-2 as previously described.2
Response and survival.
Patients with measurable disease were scored for tumor response. All sites of disease were documented at baseline radiographically and by physical examination. Disease assessments were scheduled at 6-week intervals (ie, the third week of every other course of DAB389IL-2 administration). A complete response (CR) was defined as no evidence of active disease for a duration of 4 or more weeks, with no evidence of new disease. A partial response (PR) was defined as a reduction of measurable disease greater than or equal to 50% for a duration of 4 or more weeks, with no evidence of new disease. The initial time to response was defined as the interval between the first dose administered to the date of the first documentation of at least a 50% reduction in disease. The duration of response was defined as the interval between the date of first response and the date of known disease progression.
RESULTS
Pathology assessment.
There were 230 patients screened for enrollment into this trial (Table 1). Of these, 109 patients were found to have tumors expressing the IL-2R: 69 (63%) expressed p55 only, six (6%) expressed p75 only, and 34 (31%) expressed both p55 and p75. Of the specimens screened, 78% of those with HD, 62% of those with CTCL, and 40% of those with NHL expressed one or both of the subunits for IL-2R. Seventy-three patients were enrolled in the study, but receptor expression was known for only 66. There were seven patients with HD enrolled before a protocol amendment requiring IL-2R determination for those patients. Of the patients screened, there were 157 patients excluded from the trial: 121 did not have IL-2R–expressing tumor and 36 did not meet other entry criteria.
Patient demographics.
The characteristics of the 73 patients enrolled in this study are listed in Table 2. There were 44 men and 29 women in this trial with a mean age of 50.7 years (range, 16 to 81). Almost half had a diagnosis of CTCL (n = 35) with the remainder having other NHL (n = 17) or HD (n = 21). The patients were heavily pretreated with a median of four previous therapies (range, 0 to 15) and 25 having received previous bone marrow transplantation.
Safety.
A total of 245 courses of DAB389IL-2 was administered in this study. The median number of courses was three with 71% of patients receiving at least two courses and 53% receiving at least three courses. Dose-limiting toxicity occurred at 31 μg/kg/day for 5 days due to reversible asthenia, fever, and nausea/vomiting occurring in four of five patients. Grade 3 and 4 toxicities possibly or probably related to DAB389IL-2 are listed in Table 3. There was a significant decrease in the adverse event severity with subsequent courses. Nearly all (84 of 91, 92%) of the Grade 3 and 4 clinical events occurred during the first or second course.
Patients who experienced wheezing, dyspnea, bronchospasm, chest tightness, or evidence of an allergic reaction considered by the investigator to be related to DAB389IL-2 were considered to have a hyposensitivity-like response. Fifteen of 73 patients (21%) had one or more of these symptoms. Nine of the 15 patients experienced these symptoms only in course 1. One patient with NHL and circulating malignant cells experienced symptoms intense enough to require discontinuation from the study. Eleven patients received additional courses after the course in which the hypersensitivity occurred; three of these patients developed mild recurrent symptoms. Hypersensitivity symptoms were responsive to treatment with antihistamines in most patients; two patients received steroids. Patients with hypersensitivity symptoms who received subsequent doses were premedicated with antihistamines.
Forty-five rashes were reported in 23 patients. The onset was in course 1 for 15 patients and in subsequent courses for eight patients. The rashes were characterized as generalized (56%), maculopapular (27%), petechial (2%), or urticarial (16%). The presence of antibody titer to DT, DAB389IL-2, or IL-2 did not predict for rash.
Hypotension was reported in 40 patients. Fourteen (19%) experienced a transient decrease in blood pressure during or shortly after DAB389IL-2 administration and 23 (32%) experienced hypotension that required treatment with fluids or was associated with edema or decreased serum albumin. Six patients (8%), all with CTCL, experienced hypotension, decreased serum albumin and edema, possibly representing a vascular leak syndrome.
The most frequent shifts in laboratory values were reversible hepatic transaminase elevations (n = 45, 62%) and decreased serum albumin (n = 63, 86%). Transaminase levels greater than five times normal (NCI Grade 3 or 4) were reported in 11 patients (15%). The frequency and severity of transaminase elevations decreased in subsequent courses.
Creatinine elevations above the normal range were reported in 13 patients (18%), with the average increase being 0.5 mg/dL. Grade 3 abnormal urinalysis (white blood cell [WBC]>81/high-power field [HPF]; red blood cell [RBC]>50/HPF) with or without casts was noted in 15 patients (21%) with six of these patients having minor increases in creatinine.
Four patients died within 2 months of participation in this trial. Three deaths were due to progressive disease and one was due to pneumonia.
Pharmacology.
The pharmacokinetic parameters as measured by ELISA for DAB389IL-2 are shown in Table4. Serum concentrations were measured over a 10-fold range of dose levels (3 to 31 μg/kg/day). There was a monophasic clearance of DAB389IL-2 with an overall half-life of 72 minutes. The clearance profiles on course 1, day 1 and day 5, for patients at each dose level are superimposable, indicating that DAB389IL-2 does not accumulate with repeated administration. In addition, it also appears that clearance mechanisms were not saturated over the dose range examined. Total exposure, as measured by area under the curve (AUC), was proportional to the dose administered.
Antibody evaluation.
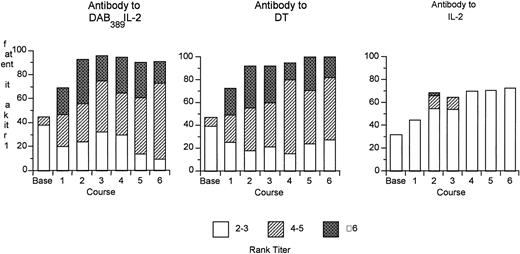
Seventy-one of the 73 patients were screened for antibodies to DAB389IL-2, DT, and IL-2 before treatment. In addition, serum samples were obtained on day 21, or before treatment on the subsequent course, for determination of titers posttreatment. Antibody assay results are expressed in rank titers representing the greatest dilution at which the specimen was reactive. Twenty-seven of the patients (38%) had detectable titers of antibody at baseline, and 92% had detectable titers after two courses. Maximum titers occurred after two courses with no further increase despite continued DAB389IL-2 administration (Fig1). There was no apparent relationship with the dose of DAB389IL-2 administered and the peak antibody titer. Anti-DT antibody titers paralleled the anti-DAB389IL-2 levels. Almost one third of patients had detectable titers to IL-2 at baseline and almost two thirds had detectable, but usually low, titers of anti–IL-2 antibody after two courses. The effect of antibody concentration on circulating levels of DAB389IL-2 was variable and probably dependent on the nature and type of antibodies involved. In some patients, high levels of antibody had no effect on the detection of circulating levels of DAB389IL-2. In others, low levels of DAB389IL-2 were measured despite the presence of very low antibody titers.
Percent of patients with rank titers for antibodies to DAB389IL-2, DT, and IL-2 by course. Relative rank titers are shown with the following designations: rank titer 1, dilution, 1:5; rank titer, 2 to 3, dilution, 1:25 to 1:125; rank titer, 4 to 5, dilution, 1:625 to 1:3125; rank titer, 6, dilution, 1:15625.
Percent of patients with rank titers for antibodies to DAB389IL-2, DT, and IL-2 by course. Relative rank titers are shown with the following designations: rank titer 1, dilution, 1:5; rank titer, 2 to 3, dilution, 1:25 to 1:125; rank titer, 4 to 5, dilution, 1:625 to 1:3125; rank titer, 6, dilution, 1:15625.
The presence of antibodies did not preclude a response. The same proportion of patients with and without a response had significant titers of antibody to DAB389IL-2, DT, or IL-2 at baseline. Similarly, the same proportion of patients with and without a response had increased antibody titers after two courses of DAB389IL-2 administration, the time when significant antitumor effects were documented.
Soluble IL-2R.
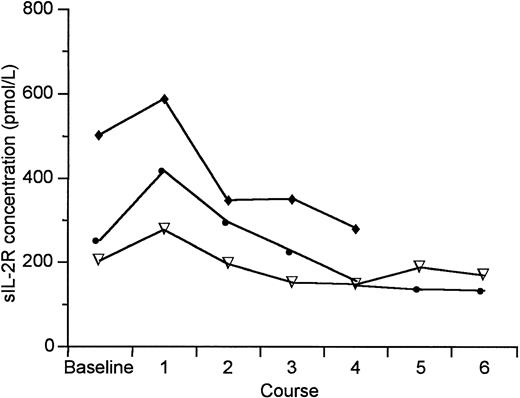
Of the 73 enrolled patients, baseline data for soluble IL-2R (sIL-2R) is available for 67 patients. Thirty-seven patients (55%) had levels that were greater than 150 pmol/L. There was generally a decrease in the mean sIL-2R level over the first two courses (Fig 2). Patients with HD had the lowest sIL-2R levels at enrollment (331 ± 265 pmol/L) at entry. The CTCL patients who did not respond to DAB389IL-2 had significantly higher levels of sIL-2R than those patients who experienced antitumor response (2,152 ± 6,975 v 254 ± 206; P = .0125, Wilcoxon Rank Sum Test). This same observation did not hold for patients with NHL (889 ± 884 v388 ± 270; P = .4469 Wilcoxon Rank Sum Test). For NHL patients, there was no significant change from baseline in the mean final sIL-2R level in the patients with clinical response (final level, 497 ± 535; P = 1.0, Wilcoxon Signed Rank Test for the change), but the mean levels for nonresponders increased significantly by 50% (final level, 1,364 ± 1,619 pmol/L; P = .0488 Wilcoxon Signed Rank Test for the change).
Median sIL-2R levels for all patients by diagnosis before and after DAB389IL-2 administration. (▿) HD; (•) CTCL; (⧫) NHL.
Median sIL-2R levels for all patients by diagnosis before and after DAB389IL-2 administration. (▿) HD; (•) CTCL; (⧫) NHL.
Peripheral blood lymphocyte evaluation.
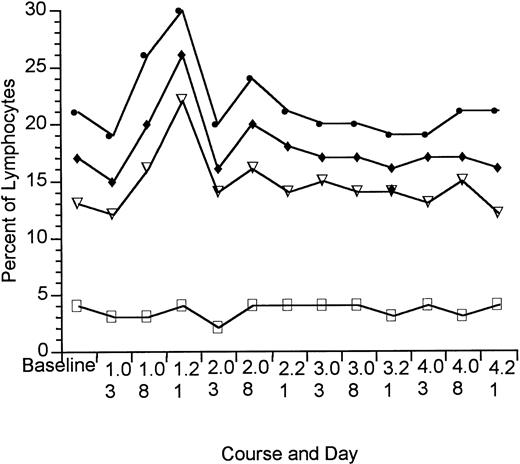
Circulating malignant cells were not detected in any of the patients with HD, but were found in six of the patients with NHL and 27 of the patients with CTCL. There was no change in the distribution of normal peripheral blood lymphocyte subset with DAB389IL-2 administration. The percent of all lymphocytes expressing CD25 (IL-2R) and the percent of T-lymphocyte subsets coexpressing CD25 are shown in Fig 3. There was an increase in overall T-cell expression of CD25 after DAB389IL-2 administration in course 1. There was no change, however, in the proportion of lymphocytes coexpressing CD3, CD4, or CD8 and CD25.
Percent of peripheral blood lymphocytes expressing CD25 before and after DAB389IL-2 administration (patients without circulating malignant cells, HD = 21; NHL = 11; CTCL = 8). (•) CD25; (⧫) CD3/25; (▿) CD4/25; (□) CD8/25.
Percent of peripheral blood lymphocytes expressing CD25 before and after DAB389IL-2 administration (patients without circulating malignant cells, HD = 21; NHL = 11; CTCL = 8). (•) CD25; (⧫) CD3/25; (▿) CD4/25; (□) CD8/25.
Response.
There were 16 patients who experienced tumor response (Table 5). For patients with CTCL, there were five CRs and eight PRs (response rate, 37%; 95% confidence interval [CI] 21% to 53%). Three of the 17 patients with NHL had tumor regression including two PRs in patients with follicular lymphomas and one CR in a patient with a diffuse large cell NHL. This latter patient had been refractory to primary chemotherapy and relapsed less than 100 days after autologous bone marrow transplant. The median time to response was 2 months and the median duration of response was 10 months (range, 2.4 to 39+). The patient with diffuse large cell NHL remains in CR at 39+ months; three patients with CTCL in CR have relapsed (10, 12, and 17 months) and two CTCL patients in CR have been lost to follow-up at last contact 15 and 22 months in remission. Responses occurred at all dose groups except the highest. There was no obvious correlation between density of the p55 and/or p75 subunit of the IL-2R and response.
DISCUSSION
The rational design of new treatment modalities in cancer therapy must emphasize agents with a novel mechanism of action, which can overcome tumor resistance, are selective for the tumor cells to minimize toxicity, and address the systemic nature of these diseases. DAB389IL-2, is an IL-2R–specific ligand fusion-protein that potentially displays these characteristics. The feasibility of using such targeted therapy was supported by experience with an earlier version of this agent, DAB486IL-2, in patients with advanced hematologic malignancies.2-7DAB389IL-2 has a fivefold improvement in affinity for the high-affinity receptor for IL-2 compared with the earlier molecule and has a 10-fold increase in potency and an extended half-life in vitro. The improvement in affinity resulted from the deletion of 97 amino acids from the fusion protein. The patients entered on this study had HD, CTCL, or NHL expressing the IL-2R. The receptor for IL-2 is complex, containing multiple subunits. The development of methodologies to detect the high-affinity IL-2R suitable for application to a clinical trial such as this one has been difficult.2,5 7 In this trial, patients were screened by confirming the histology and by assaying for the p55 and p75 subunits of the IL-2R. Overall, almost two thirds of patients screened expressed one or both of these subunits. There was not a clear relationship between expression of either subunit and response. This may be due to deficiencies in sensitivity and specificity of the IL-2R assays, variation in affinity of DAB389IL-2 for the IL-2R, variation in ability of the receptor to internalize the fusion-protein, or the impact of other variables such as tumor burden.
A diverse group of patients was enrolled in the study. The mean age of almost 51 years actually reflects a broad span, with both old and young patients tolerating the ligand fusion-protein therapy well. The patients were heavily pretreated and more than a third had received prior bone marrow transplantation. The ability to induce tumor response in such heavily treated patients suggests that DAB389IL-2 may complement existing agents in the treatment of lymphoma.
The safety profile of DAB389IL-2 does differ somewhat from the earlier DAB486IL-2, where changes in hepatic transaminases were dose-limiting.2 The present study identified asthenia as dose-limiting with significantly fewer hepatic changes. A constellation of symptoms including hypotension, edema, and decreased albumin was identified. These symptoms suggest a vascular leak syndrome, which had not been appreciated previously, but was noted only in patients with CTCL. Another finding not seen in earlier studies was the occurrence of rash. These were generally self-limited and did not correlate with antibody titer. There was no apparent effect on the immune system as evidenced by the absence of unexpected opportunistic infections or by changes in peripheral blood lymphocyte profiles.
The pharmacologic profile of DAB389IL-2 was different from DAB486IL-2.2 5 The half-life of 72 minutes was significantly longer than the 5 minutes reported for the previous construct. The agents were similar, however, in that there was no accumulation with repeated dosing and the total exposure was proportional to the dose administered.
As we had seen previously, less than 40% of patients had detectable titers of anti-DT or anti-DAB389IL-2 antibodies at study entry. After two cycles of therapy, antibody titers could be detected in almost all patients. There was no apparent relationship between dose or dose level and peak antibody titer. Neither did the presence of antibodies preclude the opportunity for tumor response, suggesting that not all DAB389IL-2 antibodies can effectively neutralize its activity. Similarly, no association was noted between the impact of preexisting antibody to DAB389IL-2, DT, or IL-2 and the occurrence of any adverse event, with the exception of hypoalbumenemia. There was a significant association of antibody titers to DT and DAB389IL-2 and the subsequent development of hypoalbuminemia (P < .05) in course 1. Additionally, a significant anti–IL-2 titer at baseline correlated with fewer episodes of nausea/vomiting, fever, chills, or rash (P < .05).
As previously reported, the presence of sIL-2R did not prevent antitumor response.2 In in vitro experiments, sIL-2R at levels < 5,000 pmol/L had no impact on the cytotoxicity of DAB389IL-2 on target cells suggesting that sIL-2R competes poorly for this agent with the IL-2R.
Although this was a phase I trial, encouraging antitumor activity was seen across a variety of dose levels. Thirty-seven percent of patients with CTCL experienced a response, with five patients achieving CR. Three patients with NHL had tumor responses, although none of the four patients with high-grade lymphomas responded. Two of the responding patients with follicular lymphomas achieved PR. One patient with intermediate grade NHL achieved CR. This patient had a tumor that was primarily refractory to chemotherapy and progressed 3 months after autologous bone marrow transplant. She remains free of disease greater than 39 months after therapy. Responses were seen in all stages of disease except for stage 4, suggesting that tumor burden may be a determinate of response. Responses were also observed in patients who had received as many as six different prior therapeutic modalities. Of interest, no patient with HD experienced a tumor response. This was an unexpected finding as patients with HD receiving DAB486IL-2 had demonstrated tumor responses and because of the high degree of expression of p55 alone and p75 subunits of the IL-2R by Hodgkin's cells.
The present report describes a clinical study of an agent redesigned through recombinant methodologies to improve its therapeutic profile. It illustrates the feasibility of using such technologies to design rational therapies of cancer. The study also illustrates the importance of correlative studies in understanding not only the biology of the tumor, but also the biology of the host in response to novel therapies.
ACKNOWLEDGMENT
The authors wish to thank Karen Parker for her technical support and Shannon Dixon for her clerical assistance.
Supported by Seragen, Inc, Hopkinton, MA.
Address reprints to C.F. LeMaistre, MD, South Texas Cancer Institute, 7700 Floyd Curl Dr, San Antonio, TX 78229.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.