Abstract
The interest in the use of human dendritic cells in cancer immunotherapy calls for efficient ex vivo methods of dendritic cell education. To extend the range of methods available, we generated phenotypically characteristic dendritic cells from peripheral blood monocytes incubated with granulocyte-macrophage colony-stimulating factor and interleukin-4 and infected them with an adenovirus containing a humanized version of green fluorescent protein as a marker of gene expression. The levels of expressed protein were high, but they were further increased in combination with cationic liposomes. In comparison to transfection efficiency of the homologous expression plasmid, adenovirus-mediated gene transfer was substantially more efficient. With the aid of liposome-mediated infection, gene transfer into CD83+ dendritic cells was highly effective, resulting in more than 90% of the cells expressing the transgene.
DENDRITIC CELLS process intracellular and internalized antigens and present them to the naive T cells.1 In vivo suppression of dendritic cell function plays a role in pathogenesis of cancer2 and infectious diseases, including acquired immunodeficiency syndrome.3,4To overcome this suppression, ex vivo maturation of dendritic cell precursors in the presence of antigen(s) has been explored as part of an immunotherapeutic approach to malignancy.5-10 Generally, isolated dendritic cells have been incubated in vitro (pulsed) with tumor-specific peptides5,7; preliminary reports on the effectiveness of this technique have been encouraging.5,7,11 12
Modification of gene expression in dendritic cells could provide important advantages over peptide pulsing. For example, expression of a transgene could extend the duration of antigen presentation. Also, expression of transgenes for cytokines and chemokines, or for these molecules fused with target peptides, could induce more potent immune responses than peptides alone. Such fused cytokines and peptides have been used as vaccines for B-cell lymphoma.10
Unlike somatic gene therapy that generally requires stable long-term expression, education of dendritic cells for antigen presentation in immunotherapy could benefit from a burst of transgene expression that occurs in parallel with ex vivo dendritic cell processing. The hitherto modes of gene delivery to dendritic cells include high-speed in vivo delivery of naked DNA bound to microspheres,13 transfection with plasmid constructs,14 and transduction with recombinant retroviruses.15,16 These techniques have shown delivery and expression of transgenes, but they have been more compatible with the requirements of somatic gene therapy. Therefore, for efficient DNA delivery to dendritic cells, as well as the favorable kinetics of transgene expression in infected cells, we considered recombinant adenoviruses. These viruses have been used successfully as vectors for DNA transfer primarily into endothelial and epithelial cells17,18 and recently into dendritic cells.19Compared with other methods, adenovirus-mediated transfer stimulated the strongest antigen-specific cytotoxic T-lymphocyte (CTL) response in mice and provided protective immunity to a lethal antigen challenge.20 21
Recent advances in isolation and culture of dendritic cells from circulating mononuclear cell precursors make it possible to prepare dendritic cells in numbers useful for clinical trials.22-25These cells are equivalent to mature blood-derived dendritic cells in phenotype, morphology, and function.22 Availability of these cells necessitates development of highly efficientmethods of gene transfer that would not compromise the later use of the cells in humans. The lack of adenovirus integration into the genome and the high level of transgene expression make this technique worth considering in ex vivo antigen education of dendritic cells for therapy of malignant and infectious diseases. In this communication, we report the use of a recombinant adenovirus in combination with cationic liposomes for highly effective gene transfer and expression in human dendritic cells.
MATERIALS AND METHODS
Cell culture media and recombinant cytokines.
In all experiments, we used the complete medium, RPMI-1640 supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from GIBCO BRL, Gaithersburg, MD). Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor-α (TNF-α) were obtained from R&D Systems (Minneapolis, MN). Cells were incubated in a water-vapor saturated atmosphere containing 5% CO2 at 37°C.
Isolation of dendritic cells.
Dendritic cells were derived from monocytes isolated by immunomagnetic adsorption26 and cultured essentially as described by others.25 Buffy coat, derived from 1 U of whole blood drawn from a healthy volunteer, was supplied by the Components Laboratory, Department of Transfusion Medicine, Mayo Clinic, in compliance with institutional guidelines. The cells, suspended in 50 mL of citrated plasma, were mixed with an equal volume of phosphate-buffered saline (PBS). The cell suspension (in aliquots of 8 mL) was layered onto 6 mL of Lymphocyte Separation Medium (Organon Teknika, Durham, NC) into 12 15-mL conical tubes. The tubes were centrifuged at 400g for 30 minutes at room temperature (all centrifugation was performed at room temperature unless noted). The cells layered between the phases were aspirated and combined into 50 mL conical tubes, washed twice with 50 mL of PBS, and centrifuged at 250g for 10 minutes. The cells were washed with 50 mL cold PBSFE (PBS with 0.5% bovine serum albumin and 2 mmol/L EDTA), counted, and assayed for viability by trypan blue exclusion.
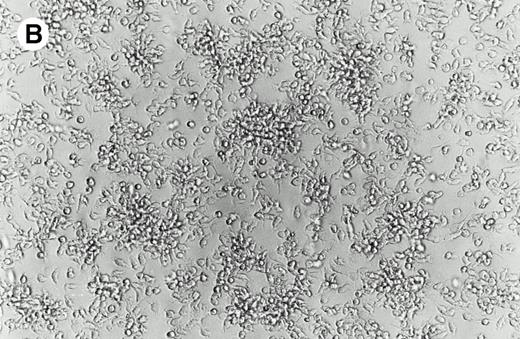
Peripheral blood mononuclear cells (5 × 108 total cells) were used as the source for immunomagnetic isolation of CD14+ leukocytes according to the manufacturer's instructions (Miltenyi, Auburn, CA). CD14+ cells (8 × 107) were resuspended at 5 × 106 cells/mL in serum-free RPMI-1640 for 1 hour at 37°C. The nonadherent cells were discarded and the attached cells were rinsed with PBS (Fig 1A). Complete medium was supplemented with 1,000 IU/mL of GM-CSF and 1,000 IU/mL IL-4. Within 48 hours, loosely attached grapelike clusters formed with some remaining adherent cells (Fig 1B). Two days after plating with cytokines, the cells were suspended at 2 × 106 cells/mL of complete medium with GM-CSF and IL-4 and incubated for an additional 72 hours. Cells were harvested by vigorous pipetting, counted, and used for flow cytometry or gene transfer. At this stage, cells were more than 92% viable as determined by trypan blue exclusion.
Morphology of isolated cells in culture. (A) Purified adherent monocytes used for initiation of dendritic cells. (B) After 48 hours in culture with GM-CSF and IL-4, monocytes clustered into aggregates typical of dendritic cell precursors.
Morphology of isolated cells in culture. (A) Purified adherent monocytes used for initiation of dendritic cells. (B) After 48 hours in culture with GM-CSF and IL-4, monocytes clustered into aggregates typical of dendritic cell precursors.
Adenovirus-mediated gene transfer.
For gene transfer, we used the recombinant adenovirus Ad5RSVGFP alone, Ad5RSVGFP in combination with cationic liposomes,27 the expression plasmid pRSVGFP containing the adenovirus expression cassette, and pRSVGFP with cationic liposomes. Ad5RSVGFP contained a humanized version of the green fluorescent protein (GFP; GFP-S65T; Clontech, Palo Alto, CA) under the control of the Rous Sarcoma virus (RSV) promoter. Ad5RSVGFP and pRSVGFP were provided by the University of Iowa Gene Transfer Vector Core (Iowa City, IA). The adenovirus contained 1.1 × 109 particles/μL and was stored at −70°C until needed.
For infection, AdRSVGFP was prepared by suspending 7.5 × 109 particles (5,000 particles per cell) in 50 μL total serum free medium (Opti-MEM; GIBCO BRL). Meanwhile, 1.25 μg cationic liposomes (Lipofectamine; GIBCO BRL) were suspended in 50 μL serum-free medium. For experiments without liposomes, the adenovirus was suspended in 100 μL serum-free medium. For plasmid transfections, we used the equivalent cell numbers, liposome amounts, and virus expression equivalents (5,000 plasmids per cell) as for Ad5RSVGFP infection. The vector and liposome solutions were mixed and incubated for 15 minutes.
For gene tranfer experiments, purified dendritic cells were centrifuged at 250g for 10 minutes and the medium was carefully removed. The cells (1.5 × 106 cells for each trial) were resuspended in 100 μL of serum-free medium and combined with the vector or vector/liposome. The suspension was gently mixed and incubated for 105 minutes at 37°C. (The final adenovirus/cell ratio was 5,000 virus particles per cell, resulting in a multiplicity of infection factor of about 50.) After incubation, the cells were centrifuged, the supernatant was removed, and the cells were resuspended at 0.5 × 106 cells/mL in complete medium containing 400 IU/mL GM-CSF and 200 IU/mL TNF-α. The cells were incubated at 37°C for 72 hours before analysis for GFP expression by flow cytometry and fluorescence microscopy.
Flow cytometric characterization of dendritic cells.
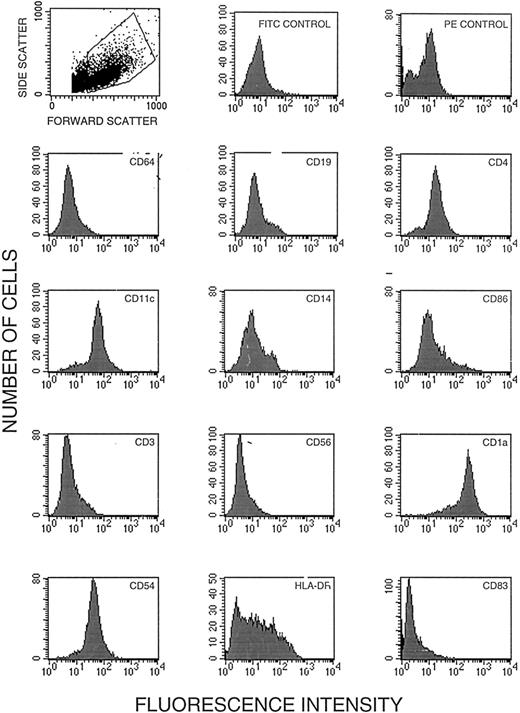
The purity of dendritic cells was assessed by flow cytometry with the aid of a FACScan cytometer (Becton Dickinson, Sunnyvale, CA) at the Mayo Flow Cytometry Core Facility. All immunoreagents were obtained commercially and were used with appropriate isotype controls. The reagent for CD56 was obtained from Becton Dickinson (San Jose, CA); for CD14, CD19, CD1a, CD54, CD3, CD4, CD11c, fluorescein isothiocyanate (FITC) isotype control, phycoerythrin (PE) isotype control, and HLA-DR from BioSource International (Camarillo, CA); for CD83 and CD64 from Coulter (Hialeah, FL); and for CD86 from Ancell (Bayport, MN). For each analysis, 100,000 cells in 100 μL PBS were incubated for 30 minutes on ice with the manufacturer's recommended amount of PE-labeled or FITC-labeled antibody. After incubation, the cells were washed with 3 mL of 0.1% sodium azide in PBS and centrifuged and the supernatant was removed. The cells were fixed with 500 μL of 2% paraformaldehyde in PBS and 10,000 events were analyzed for surface phenotype by flow cytometry (Fig 2).
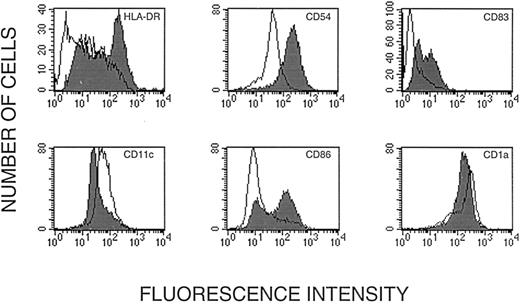
Flow cytometric analysis of human dendritic cells cultured from CD14+ leukocytes. (A) Status of 12 markers on dendritic cells after incubation with GM-CSF and IL-4; these cells were subsequently used for gene transfer. Cells in the dot plot (upper row left) were gated for homogeneity of size (forward scatter) and shape (side scatter). (B) Status of HLA-DR, CD83, CD1a, CD54, CD11c, and CD86 after ex vivo maturation with TNF-α (shaded) shown together with the status before maturation.
Flow cytometric analysis of human dendritic cells cultured from CD14+ leukocytes. (A) Status of 12 markers on dendritic cells after incubation with GM-CSF and IL-4; these cells were subsequently used for gene transfer. Cells in the dot plot (upper row left) were gated for homogeneity of size (forward scatter) and shape (side scatter). (B) Status of HLA-DR, CD83, CD1a, CD54, CD11c, and CD86 after ex vivo maturation with TNF-α (shaded) shown together with the status before maturation.
Quantitative and qualitative characterization of expressed GFP in dendritic cells.
Expression of the GFP transgene was characterized by flow cytometry with the instrument set for fluorescein detection. Three days after infection, the majority of cells did not adhere to the substrate. These cells were washed from the plates and combined with the adherent cells collected after scraping the culture flask. The cells were centrifuged and fixed for flow cytometry as described. Alternatively, dendritic cells were visualized with an IM35 Zeiss fluorescent microscope equipped with the fluorescein filter set and photographed with ISO 200 speed Royal Gold or PJM Multispeed film (Kodak, Rochester, NY).
All experiments were performed at least in duplicate with similar results.
RESULTS
Dendritic cells were cultured from monocytes by a technique that required minimum manipulation and resulted in high yields.22-24 Positive isolation of monocytes by magnetically labeled CD14-specific antibodies increased dendritic cell purity without reducing cell yield. Starting with 5 × 108 peripheral blood mononuclear cells, we typically ended up with more than 3 × 107 dendritic cell precursors. Figure 2A shows the results of flow cytometric analysis of cells after incubation with GM-CSF and IL-4. The cells contained markers typical of dendritic cell precursors22-25,28; they were positive for HLA-DR, CD54 (ICAM), CD11c, CD86, CD4, and CD1a and negative for CD56 (natural killer [NK] cells), CD64 (monocytes/macrophages), CD3 (T cells), and CD19 (B cells). Significantly, the cells were negative for CD83, a marker of mature dendritic cells.22,28 Some dendritic cells in this system expressed CD14 upon development of the ability to adhere,23 although this has not been consistently documented.22 In addition to adherent cells, the culture contained some multicell aggregates typical of proliferating dendritic cells (Fig 1B).
Incubation with GM-CSF and TNF-α transformed the immature dendritic cells into veiled cells, strongly adherent cells with dendritic appendages, and some remaining cell clusters. Flow cytometric analysis indicated an increase in expression of CD83, HLA-DR, CD54, and CD86 (Fig 2B). Although the cells were still CD1a+ and CD11c+, the level of expression of these markers was reduced. CD83 has been characterized as a unique cell surface marker of mature dendritic cells.22 28 An analysis of cell size (forward scatter) and cell geometry (side scatter) showed a discrete population of larger and less regular cells (Fig 2A). In this discrete population, we found that more than 50% of the cells were CD83+ (Fig 2B). Significantly, more than 90% of cells in this population were also HLA-DR+ (Fig 2B). Thus, the large cells in the gated region were a population of mature dendritic cells.
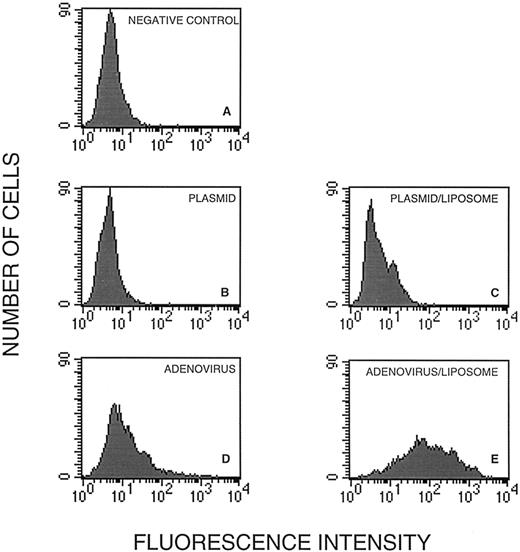
To determine the role of the recombinant adenovirus in GFP expression in dendritic cells, we transfected the cells before maturation with pRSVGFP, the plasmid contained in Ad5RSVGFP (Fig 3B), and with pRSVGFP in the presence of liposomes (Fig 3C). Also, we infected the cells with the equivalent (in comparison to pRSVGFP) amount of Ad5RSVGFP in the absence (Fig 3D) or in the presence of liposomes (Fig 3E).
GFP expression in human dendritic cells infected with pRSVGFP and Ad5RSVGFP in the absence and the presence of cationic liposomes. Cells in the gated region (Fig 2A) were analyzed for GFP fluorescence. They were untreated (A), treated with pRSVGFP in the absence (B) and in the presence of liposomes (C), or infected with Ad5RSVGFP in the absence (D) and in the presence of liposomes (E).
GFP expression in human dendritic cells infected with pRSVGFP and Ad5RSVGFP in the absence and the presence of cationic liposomes. Cells in the gated region (Fig 2A) were analyzed for GFP fluorescence. They were untreated (A), treated with pRSVGFP in the absence (B) and in the presence of liposomes (C), or infected with Ad5RSVGFP in the absence (D) and in the presence of liposomes (E).
Whereas cells treated with pRSVGFP alone (Fig 3B) were no more fluorescent that untreated controls (Fig 3A), inclusion of liposomes in the transfection medium resulted in GFP expression in some 5% of cells (Fig 3C). Interestingly, the equivalent amount of Ad5RSVGFP alone resulted in more successful gene transfer; about 20% of dendritic cells expressed GFP (Fig 3D). Adenovirus combined with liposomes was the most effective, resulting in approximately 90% of cells expressing GFP (Fig 3E). In control cells (Fig 3A) and cells transfected with the plasmid (with or without liposomes; Fig 3B and C), the mean fluorescence intensity, a measure of the average level of GFP expression per cell, was low and indistinguishable among the groups. However, cells infected with the adenovirus (Fig 3D) were characterized by the mean fluorescence intensity 2.5 times above control and the cells treated with the adenovirus and liposomes (Fig 3E) were characterized by the mean fluorescence intensity 8.5 times above control. Clearly, both adenovirus infection and liposome treatment increased the levels of transgene expression.
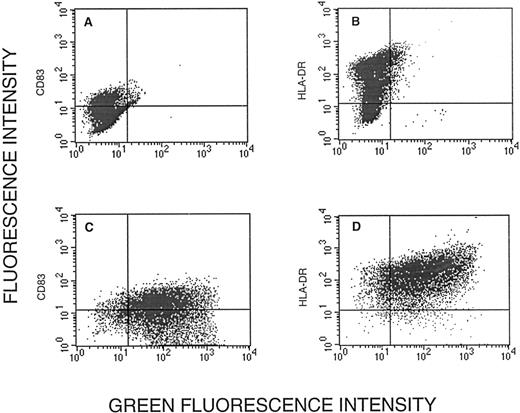
To determine whether cells expressing GFP were mature dendritic cells, we analyzed CD83 and HLA-DR fluorescence, respectively, versus GFP fluorescence. Of CD83+ cells, 90% expressed GFP (Fig 4C). Similarly, of HLA-DR+cells, some 90% expressed GFP (Fig 4D). Thus, the GFP+cells harbored the two characteristic markers of dendritic cells.
Expression of CD83 (A and C) and HLA-DR (B and D) versus green fluorescence in uninfected cells (A and B) and cells infected with Ad5RSVGFP in the presence of liposomes (C and D). The vertical and horizontal lines distinguish CD83− and HLA-DR− cells (left of and below the line, respectively) from CD83+ and HLA-DR+ cells.
Expression of CD83 (A and C) and HLA-DR (B and D) versus green fluorescence in uninfected cells (A and B) and cells infected with Ad5RSVGFP in the presence of liposomes (C and D). The vertical and horizontal lines distinguish CD83− and HLA-DR− cells (left of and below the line, respectively) from CD83+ and HLA-DR+ cells.
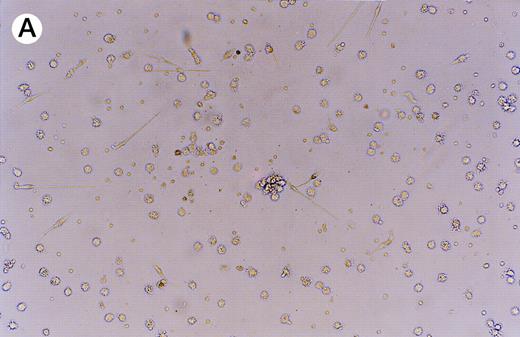
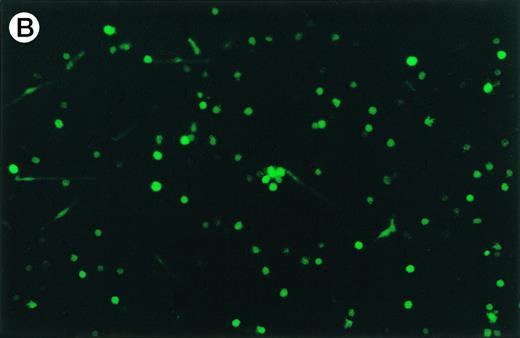
GFP expression was observed in adherent, loosely adherent, and nonadherent cells (Fig 5). The cells were morphologically typical: the unattached cells contained small pseudopodia and the attached cells displayed long bifurcated appendages. Thus, infection and transgene expression had no apparent effect on dendritic cell morphology and viability.
Expression of GFP in cultured dendritic cells. (A) Transmitted light micrograph of dendritic cells 3 days after infection with AdRSVGFP in the presence of cationic liposomes. (B) Same field as in (A) under fluorescence detection using a fluorescein filter set. GFP was expressed in adherent, nonadherent, and loosely attached cells.
Expression of GFP in cultured dendritic cells. (A) Transmitted light micrograph of dendritic cells 3 days after infection with AdRSVGFP in the presence of cationic liposomes. (B) Same field as in (A) under fluorescence detection using a fluorescein filter set. GFP was expressed in adherent, nonadherent, and loosely attached cells.
DISCUSSION
The purpose of this work was to establish a technique for high efficiency gene transfer into human dendritic cells derived from peripheral blood. We used expression of GFP as proof of infection and effective expression of the transgene. To increase the efficiency of transgene expression, we applied the recently described liposome-enhanced adenovirus infection27 to human dendritic cells. Adenoviruses alone can infect these cells, but they are rather ineffective (ie, they need high levels of multiplicity of infection19). By the use of liposomes, we dramatically increased infection efficiency.
Although the adenovirus combined with liposomes was highly infective, it was unclear whether the virus contributed to gene expression in comparison to the plasmid transfection in the presence of liposomes. To resolve the role of the adenovirus, we studied gene expression in cells transfected with the plasmid containing the adenovirus expression cassette. In cells transfected with the plasmid alone, there was virtually no transgene expression. Liposomes did induce measurable expression, but it was more than one order of magnitude below the level obtained with the adenovirus/liposome combination. Clearly, the role of the adenovirus was critical even when the transgene was transferred across the cell membrane by the liposome-mediated mechanism.27 The practical consequence of this finding is that one can achieve superinfectivity with rather low amounts of adenovirus. Thus, the high levels of gene expression achieved by the use of adenovirus in combination with liposomes make this system well suited for antigen expression and presentation.
GFP expression by cells infected by the adenovirus alone indicates that dendritic cells harbor CD51 (integrin-αv), the molecule critical for effective adenovirus infection, or its functional equivalent. In a murine dendritic cell line, GM-CSF increased the expression of CD51.21 Expression of CD51 as part of the functional integrin αv β3 and/or αv β5 raises questions about the role of vitronectin binding integrins in the biology of dendritic cells and particularly in their maturation. In mice, vitronectin receptor was found in thymocytes, splenocytes, and bone marrow cells.29In thymocytes, expression of vitronectin receptor depended on the stage of development.29 Expression of vitronectin receptors in dendritic cells may help colocalize these cells with developing T cells in the course of immune system education. This hypothesis is in line with the observation that blocking adhesion also blocks T-cell proliferation. Work is underway in our laboratory to determine the role of CD51 in human dendritic cells.
A salient feature of this work is that liposomes enhanced the adenovirus-mediated gene expression without any apparent interference with dendritic cell maturation; high levels of CD83 and HLA-DR were measured in infected cells. Because CD83 is highly expressed in adenovirus/liposome-treated cells (this work) and coexpressed with CD1a,b,c, class I, class II, CD80, and CD86, it is likely that the adenovirus/liposome-treated cells contain all the factors required for their function in immunity.
The ability of antigen-presenting dendritic cells to elicit a specific immune response has stimulated interest in the use of dendritic cells as adjuvants in immunotherapy of tumors and of autoimmune and infectious diseases. Currently, clinical trials are conducted to evaluate the usefulness of ex vivo educated dendritic cells in therapy of B-cell lymphoma7 and prostate cancer,30whereas laboratory observations promise that this treatment modality will soon be extended to other malignant diseases.31 Thus, the high efficiency gene transfer and transgene expression by dendritic cells can extend the range of potential targets for dendritic cell mediated immunotherapy. To investigate this hypothesis, we are studying antigen-dependent stimulation of the T-cell response by transgene expressing dendritic cells.
The technique that we have described is rapid and requires only a single additional manipulation step to the standard dendritic cell isolation protocol. It results in high levels of transgene expression in CD83/HLA-DR+ dendritic cells. An additional advantage of adenoviruses in ex vivo manipulation of cells for human use is their inability to integrate into the genome and change the oncogenic potential of manipulated cells. Ex vivo adenovirus-mediated gene transfer fits well into future clinical protocols for isolation and ex vivo education of dendritic cells.
ACKNOWLEDGMENT
The authors thank the Mayo Flow Cytometry Core Facility for expert assistance, Dr Beverly Davidson and Richard Anderson of the University of Iowa Vector Core Facility for the supply of pRSVGFP and Ad5RSVGFP, and Dr Franklyn G. Prendergast for his continuing interest and support.
Supported by a grant from Adelyn L. Luther, Singer Island, FL, and by the Mayo Cancer Center. A.B.D. is a Glen and Florence Voyles Foundation Scholar. The University of Iowa Gene Transfer Vector Core is supported in part by a trust from the Carver Foundation.
Address reprint requests to Allan B. Dietz, PhD, Stem Cell Laboratory, Mayo Cancer Center, Room 1311B Guggenheim, 200 First St SW, Rochester, MN 55905.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal