Abstract
We developed a simple assay for the measurement of tissue factor procoagulant activity (TF PCA) in whole blood samples that avoids the need for mononuclear cell isolation. This method combines convenience of sample collection and processing with a high degree of sensitivity and specificity for TF. Using this method, we have determined that TF PCA is detectable in whole blood samples from normal individuals, which is itself a novel observation. Essentially all PCA could be shown to be localized in the mononuclear cell fraction of blood. Compared with controls, whole blood TF levels were significantly (P < .000001) elevated in patients with sickle cell disease (SCD), regardless of the subtype of hemoglobinopathy (SS or SC disease). No significant difference in TF PCA was observed between patients in pain crisis compared with those in steady-state disease. Because TF functions as cofactor in the proteolytic conversion of FVII to FVIIa in vitro, it was expected that an increase in circulating TF PCA would lead to an increased in vivo generation of FVIIa. On the contrary, FVIIa levels were actually decreased in the plasma of patients with SCD. Plasma TF pathway inhibitor (TFPI) antigen levels were normal in SCD patients, suggesting that accelerated clearance of FVIIa by the TFPI pathway was not responsible for the reduced FVIIa levels. We propose that elevated levels of circulating TF PCA may play an important role in triggering the activation of coagulation known to occur in patients with SCD. Because TF is the principal cellular ligand for FVIIa, it is possible that increased binding to TF accounts for the diminished plasma FVIIa levels.
THE CLINICAL COURSE of sickle cell disease (SCD) is punctuated by episodic vascular occlusive events. The possibility that activation of the clotting system plays a contributory role in these complications is supported by abundant clinical data indicating that activation of platelets,1,2 plasma coagulation,3,4 and fibrinolysis5 occurs during both steady-state disease and pain crisis. In some studies, the markers of activation of these pathways have been more accentuated during pain crisis compared with steady-state disease,6 although overall this trend is not consistent.7 Whether there is an increased incidence of venous thrombo-embolic disease in SCD has not been adequately evaluated, but thrombosis probably does play an important role in several other recognized complications, including stroke, acute chest syndrome, leg ulceration, and placental infarction.8 9
Tissue factor (TF) is a transmembrane glycoprotein that forms a complex with circulating FVII(a). Upon binding to TF, zymogen FVII may become a better substrate for auto-activation and perhaps activation by other serine proteases, including FIXa, FXa, FXIIa, and thrombin.10,11 Although the precise mechanism of FVII activation that operates in vivo has not been unequivocally elucidated, there is evidence that FIXa may play a pivotal role.12 The TF-VIIa complex activates factor X directly or indirectly via factor IXa generation, ultimately leading to thrombin formation. As demonstrated by immunocytochemical techniques, TF is localized to the adventitia of normal blood vessels,13,14 in which location it is believed to come into contact with blood only after vascular injury. Although TF is not constitutively expressed by endothelial cells and monocytes in vivo, it has been demonstrated that both cell types are capable of de novo TF synthesis in vitro after exposure to lipopolysaccharide (LPS; endotoxin) and other agonists, including interleukin-1,15 tumor necrosis factor,16 and C-reactive protein (CRP).17 Nonetheless, although TF is now recognized as the sole physiological initiator of hemostasis, its role in pathological thrombosis is less well established.
In a previously published study, accelerated plasma FVII turnover in patients with SCD was reported, suggesting enhanced TF expression and/or exposure of TF to circulating clotting factors in this patient population.18 Using a novel method that we have developed to assay circulating whole blood TF (WBTF) activity, we measured functional TF activity in whole blood samples from normal controls and patients with SCD. Our results suggest that quantifiable TF activity is present in normal individuals and provide confirmation that circulating TF procoagulant activity (PCA) is elevated in patients with SCD.
PATIENTS AND METHODS
Patients.
Blood samples were collected from patients with both HbSS and HbSC disease after the subjects or their guardians signed a consent form that had been previously approved by the respective institutional committee on the use of human subjects in research. Patient age ranged from 3 months to 49 years (median, 8.1 years), and 47% of patients were male. All samples from SCD patients were obtained at least 6 weeks after any blood transfusion or exchange transfusion. Steady state was assumed if the patient was removed from a pain crisis episode for at least 2 weeks. Blood was generally obtained from patients in the ambulatory setting for those in steady state and during hospitalization for those in acute pain crisis. Controls consisted of normal Caucasian and Black adults. A separate consent form for controls (approved by the institutional human subjects committee review board) was used whenever blood was drawn. Care was taken to obtain specimens by clean venipuncture, after the first 3 mL of blood had been discarded.
Preparation of phosphatidylserine (PS):phosphatidylcholine (PC) vesicles.
Unilamellar vesicles containing 70% PC:30% PS by weight were prepared as previously described.19 Briefly, Tris-buffered saline (TBS; 0.1 mol/L NaCl, 0.05 mol/L Tris, pH 7.5), supplemented with 375 mmol/L Octyl-β-D-glucopyranoside (Calbiochem, La Jolla, CA), was added to egg yolk PC and bovine brain PS (Sigma, St Louis, MO) to yield a final phospholipid stock concentration of 25 mmol/L. Vigorous vortexing was necessary to completely solublize the phospholipids. PS and PC stocks were added to yield the desired phospholipid vesicle ratio (by weight) of 30:70. Nitrogen exposure was maintained throughout the procedure to minimize any potential phospholipid oxidation. Vesicles were dialyzed into degassed buffers of TBS for 48 hours followed by HEPES-buffered saline (HBS; 137 mmol/L NaCl, 5.38 mmol/L KCl, 5.55 mmol/L glucose, 10 mmol/L HEPES, pH 7.5) for 24 hours at 4°C and stored under nitrogen at −70°C.
Sample processing for assay of WBTF PCA.
Blood was routinely collected into vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) containing EDTA using a 21-gauge needle. In some control experiments, blood was also drawn into tubes containing 3.2% trisodium citrate or heparin as anticoagulant. Anticoagulated samples were then transfered immediately to polypropylene tubes and frozen at −70°C until TF PCA could be assayed. Batched samples were subjected to 3 cycles of freezing on dry ice followed by thawing at 37°C to completely lyse all cellular elements. One hundred microliters of lysed blood and 900 μL TBS-0.1 mol/L EDTA, pH 7.5, was then centrifuged at 450,000g for 15 minutes in a Beckman TL100 ultracentrifuge (Beckman, Palo Alto, CA) to pellet all particulate material (organelles and membrane fragments/vesicles). Control experiments in which the dilution factor of the sample and centrifugation times were varied were performed to optimize conditions. Final conditions described for the assay were those that maximized measurable PCA.
After aspiration of the supernatant, the membrane pellet was resuspended in TBS-EDTA buffer and the washing/centrifugation cycle was repeated twice. The membrane pellet was then washed one further time in 900 μL of HEPES buffered salt solution containing 0.1% bovine serum albumin (HBSA). The sample was then resuspended in 100 μL HBSA for assay of TF PCA, which was performed using the two-stage clotting assay.
Two-stage clotting assay for TF.
In the first stage of the assay, 20 μL of the diluted sample was mixed with 5 nmol/L recombinant FVIIa, 250 nmol/L human factor X (both from Calbiochem), and 8.3 mmol/L CaCl2 in HBSA (final volume, 60 μL). After 3 minutes of incubation at 37°C, 100 μL of bovine plasma (Irvine Scientific, Santa Anna, CA) containing PS 30:PC 70 vesicles (12.5 μmol/L) and HEPES (50 mmol/L) together with 100 μL 25 mmol/L CaCl2 was added. The clotting end-point was determined in a Coag-a-mate optical end point instrument (Organon Teknika, Durham, NC).
A standard curve was constructed using relipidated human brain TF. Human brain TF apoprotein prepared as previously described was reconstituted into phospholipid vesicles containing 70% PC and 30% PS.19 By definition, 1 pg of the standard gave 1 U of TF PCA. A log-log plot of TF concentration versus clot time was linear in the 1 to 1,000 pg/mL (1 to 1,000 U/mL) range, with a correlation coefficient (r) value of .996.
Demonstration of TF PCA in the mononuclear cell (MNC) fraction of whole blood.
To heparin-anticoagulated whole blood ex vivo, either LPS (1 μg/mL; Sigma) or buffer (HEPES-buffered salt solution) control was added. Samples were then incubated at 37°C for 6 hours to allow TF induction in the LPS-treated tubes. The MNC fractions were then isolated by layering the blood over Histopaque-1077 (Sigma) and centrifuging (400g) at 24°C for 30 minutes. The MNCs collected from the plasma/Histopaque interface were then washed three times with phosphate-buffered saline (PBS) and finally resuspended in 1 mL of HBSA. MNCs were then subjected to three freeze-thaw cycles, and 1/10 dilution of the lysate was suspended in HBSA before assay of TF PCA. Parallel whole blood samples that had been exposed to LPS or the control buffer were immediately freeze-thawed at the end of the 6-hour incubation period without the additional MNC isolation step. These samples were then processed according to the method for measuring WBTF PCA described above. After appropriate adjustment of the raw data according to dilutions that had been made, the total amount of TF PCA (in units per milliliter) was calculated for the whole blood and isolated MNC fraction, respectively.
Assay of TF PCA in intact cellular fraction of peripheral blood.
We used isolated intact peripheral blood cellular fractions to confirm that TF PCA measured by the whole blood method is an accurate representation of functional cell surface-expressed TF activity. In these experiments, platelet-poor cell fractions were isolated from blood drawn into heparin-anticoagulated vacutainer tubes. The samples were centrifuged at 850g for 10 minutes, after which the supernatant (platelet-rich plasma) was removed and discarded. In some experiments, whole blood from control subjects was treated ex vivo with 1 μg/mL LPS for 6 hours at 37°C before isolation of the cellular pellets. The cell pellet was then washed 3 times in TBS with 5 mmol/L EDTA to remove any remaining contamination with soluble plasma components. After a further two washes in TBS, cells were then resuspended in TBS at a 30% hematocrit. A modified two-stage assay for TF PCA was then performed in a Stago ST4 coagulometer (Diagnostica Stago, Parsippany, NJ). Recombinant FVIIa (5 nmol/L), human FX (250 nmol/L), and CaCl2 (8.3 mmol/L) were then added (final volume, 120 μL) and the mixture was incubated at 37°C for 30 minutes. One hundred microliters of bovine plasma containing PS 30:PC 70 vesicles (12.5 μmol/L) and HEPES (50 mmol/L) was then added with 100 μL of 25 mmol/L CaCl2, and the clotting time was recorded. Data are presented as the raw clotting times recorded on the coagulometer.
Plasma FVIIa assay.
Platelet-poor plasma was prepared from citrated vacutainer tubes within 1 hour after collection by centrifugation at 17,000g for 15 minutes. To avoid possible cold activation of FVII zymogen, care was taken to maintain samples at room temperature during transportation to the laboratory and throughout processing. Plasma was frozen at −70°C until batched samples could be assayed. Plasma FVIIa levels were measured as previously described, using a soluble mutant form of TF (kindly provided by Dr J. Morrissey, Oklahoma Medical Research Foundation, Oklahoma City) that is unable to facilitate conversion of FVII to FVIIa and therefore is sensitive only to FVIIa (and not zymogen FVII) in clotting assays.20Briefly, samples were thawed at 37°C and then diluted 1/10 in FVII-deficient plasma (George King Biomedical, Overland Park, KS). One hundred microliters of the soluble TF reagent (1 μmol/L soluble TF, 200 μmol/L PC 40:PE 40:PS 20 vesicles, 0.1% BSA in TBS) was incubated for 200 seconds at 37°C. After this incubation, 100 μL of the diluted plasma sample was added and incubated for an additional 30 seconds at 37°C. The reaction start time was initiated with the addition of 100 μL of prewarmed 25 mmol/L CaCl2. Clot times were determined by using the Coag-a-mate optical end point instrument and converted to nanograms per milliliter from a standard curve prepared using known concentrations of FVIIa that had been diluted in FVII-deficient plasma.
Plasma TF pathway inhibitor (TFPI) antigen, FVII antigen, and D-dimer enzyme-linked immunosorbent assay (ELISA).
Plasma TFPI antigen was measured by ELISA (American Diagnostica, Greenwich, CT) in samples from patients with SCD and from controls. Care was taken to ensure that samples were from subjects not currently receiving heparin anticoagulation, because of the known effect of heparin on increasing plasma TFPI levels.21 D-dimer was quantified also by ELISA (American Diagnostica); the quoted upper end of the normal range was 120 ng/mL. FVII antigen ELISA kits were obtained from American Bioproducts (Parsippany, NJ).
Statistical analysis.
Because several of the variables analysed appeared to have skewed distributions, logarithmic transformation of the data was performed. Unless otherwise stated, all values presented are geometric means with 95% confidence intervals. The Student's t-test was used to compare means of logarithmically transformed data. All reportedP values are two-tailed and a P value of less than .05 was considered to be significant. Spearman's rank correlation method was used to calculate correlation coefficients between variables. These values were calculated using Statistica software for the Macintosh (Statsoft, Inc, Tulsa, OK).
RESULTS
Validation of the WBTF assay as a measure of circulating TF PCA.
We determined that, at an optimal 1/10 dilution of the processed sample, clotting times fell within the linear range on a semilogarithmic standard curve plot (using relipidated human brain TF as standard). We validated that the assay is specific for TF by demonstrating that PCA was entirely dependent on the addition of FVIIa in the first stage of the clotting assay and that activity could be inhibited by more than 95% by the addition of previously described monoclonal22 or polyclonal anti-TF antibodies23(data not shown). To determine the precision of the assay, equal volumes of whole blood pellet from 20 normal or SCD samples was pooled and vigorously mixed by vortexing before repetitive assay for TF PCA on 6 separate occasions. Intrasample coefficient of variation (CV) was 5.7% for the pooled normal sample and 4.4% for the abnormal (SCD) sample. We also found that the level of WBTF PCA did not vary significantly in specimens obtained serially at a given blood draw (after the first 3 mL of blood had been discarded), indicating that contamination of the patient specimen with dermal fibroblasts or other cells known to express high levels of TF13,14 is unlikely to account for the measurable PCA. However, when samples were drawn and processed on 6 different days for 3 control subjects, a significant day to day variability was evident, yielding individual CVs of 35%, 41%, and 44%. The reason for this moderately pronounced biologic variability for a given test subject is unknown.
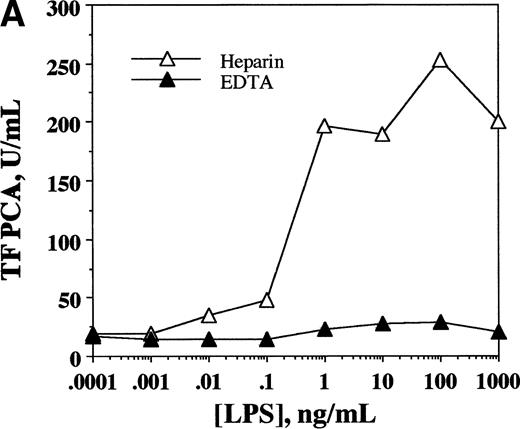
To demonstrate the inducible nature of TF PCA in whole blood, samples from a normal donor were drawn simultaneously into EDTA- or heparin-anticoagulated vacutainer tubes. To the whole blood ex vivo, LPS (an agonist expected to induce monocyte TF synthesis) was added in concentrations ranging from 0.001 to 1,000 ng/mL. Samples were then incubated at 37°C for 6 hours, after which they were transferred to polypropylene tubes, frozen at −70°C, and processed for assay of WBTF PCA, as described above. As shown in Fig 1A, the expected increase in TF PCA was evident in a dose-response relationship in heparinized samples to which LPS had been added. No such activity was inducible in EDTA samples, consistent with previous studies indicating a requirement for extracellular calcium for the optimal induction of TF.24 TF PCA was inducible in samples from both normal controls and patients with SCD that were treated with LPS ex vivo. Similar to the findings of others,25 we observed a wide variation in individual responses to LPS (from 2.4-to 9.0-fold increase), with no apparent difference in the magnitude of the response between controls and SCD patients. The fact that whole blood PCA is inducible by the addition of an agent known to induce TF in circulating monocytes lends further support to the concept that our assay is indeed measuring TF activity.
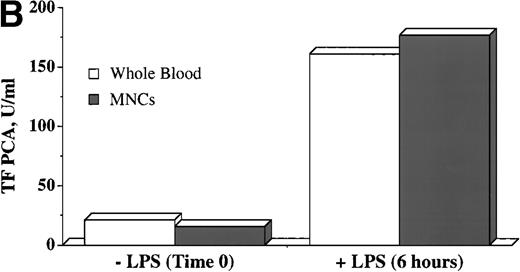
WBTF PCA may be induced ex vivo by LPS and can be recovered in the MNC fraction. (A) Samples of whole blood anticoagulated in heparin (▵) or EDTA (▴) were incubated with the indicated concentrations of LPS for 6 hours, after which they were frozen before assay of TF PCA as described in Patients and Methods. (B) WBTF PCA was measured in heparin-anticoagulated whole blood (□) both at baseline and 6 hours after incubation with LPS (1 μg/mL). In parallel samples, MNCs were isolated both at baseline and after a similar incubation with LPS. TF PCA was then assayed in the isolated MNC (▪). The data in each figure are from one experiment that is representative of four such experiments.
WBTF PCA may be induced ex vivo by LPS and can be recovered in the MNC fraction. (A) Samples of whole blood anticoagulated in heparin (▵) or EDTA (▴) were incubated with the indicated concentrations of LPS for 6 hours, after which they were frozen before assay of TF PCA as described in Patients and Methods. (B) WBTF PCA was measured in heparin-anticoagulated whole blood (□) both at baseline and 6 hours after incubation with LPS (1 μg/mL). In parallel samples, MNCs were isolated both at baseline and after a similar incubation with LPS. TF PCA was then assayed in the isolated MNC (▪). The data in each figure are from one experiment that is representative of four such experiments.
To determine what proportion of the observed PCA was to be found in the MNC fraction of whole blood from normal donors, TF PCA was assayed in the isolated (freeze-thawed) MNC fraction and compared with the value obtained by whole blood assay. It is clear from Fig 1B that values for TF PCA are essentially equivalent for both methods, both at baseline and after LPS stimulation. These data suggest that essentially all TF PCA that can be assayed by the whole blood method in normal individuals is present in the MNC fraction, but they do not necessarily establish monocytes as the sole source of this activity.
Increased WBTF PCA in normals and in patients with SCD.
As shown in Fig 2 and Table1, detectable WBTF PCA could be measured in normal persons (n = 65), with absolute values that ranged from 1.6 to 84.0 U/mL. Unlike some parameters of hemostasis that have demonstrated enhanced activation of coagulation in normal Black individuals when compared with non-Blacks,3 TF PCA did not appear to demonstrate any such racial differences. In 3 patients with high reticulocyte counts who had undergone splenectomy for hemolytic anemias, TF PCA levels were normal, with a geometric mean value of 20.2 U/mL.
WBTF PCA is elevated in patients with SCD. EDTA-anticoagulated whole blood samples from normal controls and patients with SCD were frozen at −70°C immediately after collection. WBTF PCA was measured as described in Patients and Methods. Each symbol represents a single data point; samples are included from both Black (•) and Caucasian (○) control subjects. Patient samples include those drawn during steady-state disease (▵) as well as during pain crisis (▴).
WBTF PCA is elevated in patients with SCD. EDTA-anticoagulated whole blood samples from normal controls and patients with SCD were frozen at −70°C immediately after collection. WBTF PCA was measured as described in Patients and Methods. Each symbol represents a single data point; samples are included from both Black (•) and Caucasian (○) control subjects. Patient samples include those drawn during steady-state disease (▵) as well as during pain crisis (▴).
Compared with controls, TF PCA was significantly (P < .000001) elevated for the SCD group as a whole (n = 85), with absolute values ranging from 3.1 to 438.6 U/mL. Values did not appear to differ significantly according to type of hemoglobinopathy (hemoglobin SC [n = 15] or SS [n = 70] disease) or patient age (≤16 or >16 years of age; n = 44 and 41, respectively). We hypothesized that WBTF PCA levels would be significantly elevated in pain crisis (n = 42) compared with steady-state disease (n = 43). Surprisingly, however, no such association was seen. In several patients with SCD, in whom multiple samples were available for assay, the status with respect to pain crisis did not appear to be a primary determinant of the absolute magnitude of WBTF PCA. Despite the absence of such an association, the intersample variability in TF PCA was notably more pronounced than in normal subjects; eg, in 3 patients with SCD in whom 6 or more samples were available, individual CVs were 119%, 88%, and 59%.
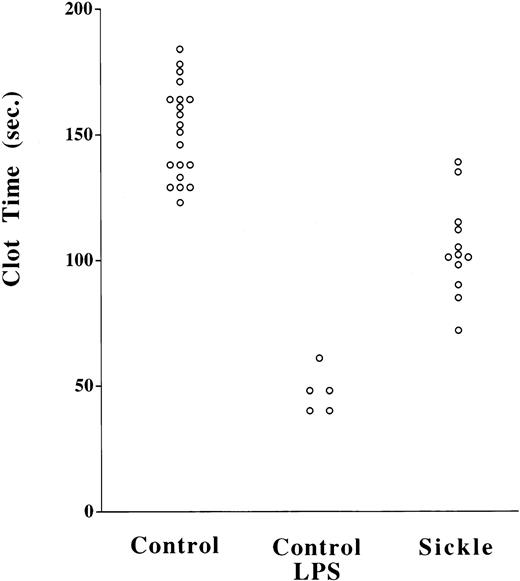
We measured PCA of intact peripheral blood cell preparations to confirm that the WBTF method is an accurate reflection of functional TF on living cells. Because these samples contained red blood cells and leukocytes, we chose to measure clotting times in the ST4 coagulometer, which does not rely on an optical endpoint. As shown in Fig 3, clotting times were significantly shorter in LPS-treated control samples compared with their untreated counterparts (P < .0005). This suggests that the PCA measured in this assay, like that in the whole blood assay, is LPS-inducible, a feature that favors that it is indeed due to TF. Furthermore, the activity could be abrogated by 2 μg/mL of a specific polyclonal antibody to TF23; eg, in one representative experiment, the mean clotting time of an LPS-treated sample increased from 48.7 to 129.2 seconds with the addition of the antibody. This PCA was also shown to be largely FVIIa-dependent, such that, when FVIIa was omitted, clotting times were typically greater than 300 seconds in controls and 95 to 100 seconds in LPS-treated samples. Clotting times in SCD samples (n = 12) were significantly (P < .000001) shorter than controls (n = 20). This is therefore indicative of a more pronounced PCA in SCD patients and is consistent with the data obtained from TF functional assay of lysed whole blood samples.
Increased TF PCA in intact cellular fractions in SCD. Platelet-depleted cell fractions were isolated by centifugation from heparin-anticoagulated whole blood. PCA was measured on these washed intact (non–freeze-thawed) cell fractions using a two-stage assay as described in Patients and Methods. P < .0005 for control versus LPS-treated controls; P < .000001 for control versus SCD.
Increased TF PCA in intact cellular fractions in SCD. Platelet-depleted cell fractions were isolated by centifugation from heparin-anticoagulated whole blood. PCA was measured on these washed intact (non–freeze-thawed) cell fractions using a two-stage assay as described in Patients and Methods. P < .0005 for control versus LPS-treated controls; P < .000001 for control versus SCD.
Because absolute monocyte counts are known to be elevated in patients with SCD,26 we considered the possibility that increased WBTF levels might simply be a function of monocytosis. Unfortunately, monocyte counts were not available for all patient samples in which TF PCA was analyzed. However, in a subset of 34 patients, although absolute monocyte counts were somewhat elevated (1.15 ± 1.05 × 109/L [mean ± SD]), no correlation existed between the count and the measured WBTF PCA (r = .012;P = .95). This result suggests that the number of functional TF molecules per monocyte is quite variable or, alternatively, that some other circulating cell type (or fragment thereof) contributes to the total pool of TF PCA in patients with SCD.
Plasma FVIIa levels are decreased in patients with SCD.
In vitro studies suggest that TF acts as cofactor for the proteolytic activation of zymogen FVII.11 In normal individuals, it has been shown that approximately 1% of the circulating plasma pool of FVII is present in the activated form.20,27 We therefore hypothesized that elevation of WBTF PCA would lead to accelerated activation of FVII, with a resultant increase in plasma [FVIIa] in patients with SCD. Using a recently described assay that measures only the activated form of FVII in plasma,20 we were therefore surprised to find the opposite result, namely, significantly diminished levels of FVIIa in SCD (Fig 4 and Table 1). As shown, normal controls (n = 55) had plasma [FVIIa] that were similar to previously published values.20 27 Patients with SCD (n = 34) had levels that were significantly reduced (P < .0005 compared with controls). Plasma [FVIIa] did not differ significantly for children ≤16 years of age (n = 13) compared with adults (n = 21), and the levels did not differ between patients according to whether their hemoglobinopathy subtype was SS (n = 27) or SC (n = 7) disease. Although plasma [FVIIa] levels overlapped to a greater degree between patients in crisis (n = 15) compared with those in steady-state disease (n = 19), we did observe somewhat higher levels during crisis. Despite the fact that plasma [FVIIa] appeared to be approximately inversely related to TF PCA, the correlation was not statistically significant either for controls (r = .29; P = .22) or for patients with SCD (r= .21; P = .33).
Plasma [FVIIa] is decreased in patients with SCD. Citrated plasma samples from normal controls or patients with SCD were assayed for plasma [FVIIa] as described in Patients and Methods. Each symbol represents a single data point; samples are included from both Black (•) and Caucasian (○) control subjects. Patient samples include those drawn during steady-state disease (▵) as well as during pain crisis (▴).
Plasma [FVIIa] is decreased in patients with SCD. Citrated plasma samples from normal controls or patients with SCD were assayed for plasma [FVIIa] as described in Patients and Methods. Each symbol represents a single data point; samples are included from both Black (•) and Caucasian (○) control subjects. Patient samples include those drawn during steady-state disease (▵) as well as during pain crisis (▴).
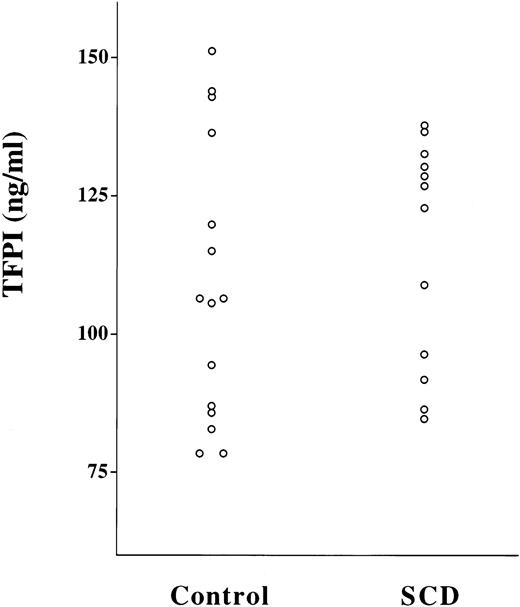
The half-life of FVIIa in vivo is significantly longer than other serine proteases of the coagulation pathway, at approximately 2.5 hours.27 This is probably accounted for by the fact that, unlike these other enzymes, free FVIIa is not rapidly inactivated by antithrombin III. However, although FVIIa bound to TF may be a target for inactivation by antithrombin III,28 it is probably principally inactivated at this site by the TFPI-FXa complex.29 To determine whether the decrement in functional plasma [FVIIa] in SCD patients could be explained by increased levels of TFPI, we assayed TFPI antigen levels in a subset of these plasmas (Fig 5). The values in the control group (107 ng/mL; range, 95 to 120 ng/mL; n = 15) were not significantly different than those for patients with SCD (114 ng/mL; range, 102 to 126 ng/mL; n = 12; P = not significant [NS]). It also seems unlikely that the extremely low plasma FVIIa levels that we observed in SCD are simply a reflection of a low total circulating mass of FVII. In a subset of 24 patients with SCD and 24 controls, FVII antigen levels were assayed by ELISA. Although, as others have described,18 mean FVII antigen was slightly lower in SCD patients compared with controls (73% [range, 68% to 78%] v80% [range, 76% to 83%], respectively; P = .05), the magnitude of the difference is unlikely to account for the profound difference in plasma [FVIIa] that we observed.
Plasma TFPI antigen levels are normal in patients with SCD. Plasma TFPI antigen was measured by ELISA. P = NS for controls versus SCD.
Plasma TFPI antigen levels are normal in patients with SCD. Plasma TFPI antigen was measured by ELISA. P = NS for controls versus SCD.
Plasma D-dimer levels are normal in patients with SCD.
To determine whether TF PCA correlated with other parameters of activation of coagulation in vivo, we measured D-dimer levels as an index of intravascular fibrin generation in a subset of patients and controls. Similar to previously published data,3,5 we found that D-dimer levels were significantly (P < .00005) elevated in SCD patients (124 ng/mL; range, 77 to 201 ng/mL; n = 17) compared with normal controls (30 ng/mL; range, 27 to 34 ng/mL; n = 19). Moreover, levels were significantly elevated both in steady state (n = 11) and during pain crisis (n = 6) (P < .005 for each group compared with controls). Also in keeping with the results of other studies,3 D-dimer levels were increased in patients during crisis (327 ng/mL; range, 140 to 762 ng/mL) compared with those not in crisis (73 ng/mL; range, 44 to 123 ng/mL; P< .05). However, notably, no significant correlation between TF PCA and D-dimer levels was apparent for either controls (r = -.005;P = .98) or patients with SCD (r = .24; P = .36).
DISCUSSION
We have developed a novel assay for the measurement of circulating functional TF activity in whole blood samples. This assay is specific for TF and sensitive to the presence of as little as 1 pg of TF antigen per milliliter of whole blood. The sensitivity of the assay is well below our ability to detect antigen on the surface of circulating cells by fluorescence-activated cell sorting (data not shown). This may not be surprising, because even if one assumes that all TF in a sample containing (for example) 100 pg/mL of TF PCA is present exclusively on monocytes in which location it is fully functionally intact, then each cell will be expressing approximately only 300 molecules of TF. Although the precision of the assay itself appears to be good, there is apparently a significant intrinsic biologic variability, more marked in patients with SCD, the reasons for which are as yet unclear. Perhaps the greatest practical advantage offered by this method is the convenience of sample collection; in the absence of any requirement for MNC isolation, samples may be collected and frozen at −70°C until the assay can be performed. Furthermore, because no isolation of MNCs is required, the potential for artifactual increase in monocyte TF activity, which may occur ex vivo during the isolation procedure or by exposure of these cells to trace amounts of contaminating endotoxin, is absolutely avoided.
Using this assay, we have demonstrated that TF PCA is present in the blood of all normal individuals, itself a novel observation. Current opinion suggests that, although TF may be inducible in monocytes and endothelial cells, it is not constitutively expressed by blood cells in their basal state.29,30 Our data demonstrate that, in normal volunteers, essentially all basal and inducible TF activity in whole blood is present in the mononuclear cell fraction of blood, but do not prove that it is exclusively present in monocytes. However, it is reasonable to conclude that this is the case, because lymphocytes (the other major cell type in MNC fractions of whole blood) have not been shown to express TF, either constitutively or after activation. We cannot totally rule out the possibility that some other cell type, perhaps even one that is not generally considered to be a circulating peripheral blood cell, is contributing to the measurable PCA. In this regard, we have recently confirmed a previous finding31that the number of circulating endothelial cells (ECs) is increased in patients with SCD and have shown that, in contrast to ECs from normal donors, TF expression may be detected in a significant proportion of ECs obtained from patients with SCD.32 However, preliminary experiments in SCD donors in which whole blood was depleted of ECs before measurement of WBTF PCA have suggested to us that less than 10% of the total pool of circulating TF activity could be accounted for by the EC fraction (Key and Hebbel, personal observation). This result is consistent with the much greater absolute number of circulating monocytes compared with ECs.
It may be argued that, because our assay is designed to measure total TF activity in lysed cellular extracts, we could be measuring a pool of TF that, although present within the cell, is not available on the surface of intact cells to bind FVII(a) and thereby initiate coagulation. Previous studies have shown that, in most cell types (with the possible exception of some malignant clones), all cellular TF apoprotein is present on the surface of cells in which it is expressed.33 However, the fact that PCA increases after cellular disruption, eg, by repeated freeze-thawing, suggests that full functional expression of TF may be regulated by other mechanisms.34 35 The biologic significance of this regulated expression of cell surface TF PCA remains to be determined. Regardless of this issue, we found that PCA demonstrating the characteristics of TF was increased in intact cellular fractions of blood from patients with SCD when compared with controls. Thus, we are of the opinion that TF PCA measured in lysed cells in whole blood samples is an accurate reflection of the relative amounts of in vivo TF expression in health and in disease states. Nonetheless, it is likely that the mere presence of intravascular TF may not be sufficient to trigger coagulation, and cellular events that activate the full expression of TF PCA may contribute to the enhanced intravascular coagulation observed in patients with SCD.
Our data do not address the reason for increased TF expression in SCD. It is tempting to speculate that the documented elevation in SCD of plasma levels of soluble mediators known to induce TF synthesis in monocytes (including tumor necrosis factor16,36 and CRP17,37-39) may provide the stimulus for TF synthesis. We failed to document any difference between WBTF levels for patients in steady-state disease versus those in pain crisis. This may reflect the fact that the distinction between crisis and steady-state disease is probably an artificial one. Subclinical ischemic episodes, as evidenced by subjective assessment of pain and by periodic elevations in biochemical and rheological indices, occur almost constantly in what is generally considered to be the steady-state phase of the disease.37-39 In contrast to TF PCA, we did detect higher levels of FVIIa and D-dimers in patients during crisis, although the baseline values (ie, during steady-state disease) were also clearly abnormal. Reconciliation of these data suggests that TF PCA may lead to a tonic activation of coagulation, but that there may also occur a superimposed burst of increased turnover of coagulation factors coincident with the onset of crisis. We are currently pursuing this hypothesis by prospective serial evaluation of WBTF PCA, plasma [FVIIa], and D-dimers in selected patients. The trigger for the superimposed elevation of FVIIa and D-dimer over baseline with the onset of crisis may be explained by increased access to TF PCA on EC or in an extravascular site, either of which would not be detectable by our assay.
One possible explanation for the apparent paradoxical decrease in plasma [FVIIa] despite enhanced TF expression is that FVIIa undergoes an accelerated clearance from the circulation. We note that normal human volunteers who received a bolus infusion of endotoxin also developed a paradoxical decrease in plasma FVIIa levels.40In that study, FVIIa levels began to decrease within the first 2 hours after endotoxin administration, but did not reach a nadir until 12 to 24 hours. Although not reported, based on previous studies it seems reasonable to assume that peak expression of TF in monocytes (and possibly endothelial cells) occurred at an earlier time point, perhaps after 6 to 8 hours. Similarly, Mesters et al41recently described that a rapid decrease in plasma [FVIIa] occurs in neutropenic patients during sepsis, particularly when associated with septic shock. In considering mechanisms to account for this phenomenon, we measured plasma TFPI antigen levels, but found no decrease in patients with SCD. It should be noted that TFPI levels have also been found to be normal or elevated in other clinical conditions in which there is evidence for TF-initiated thrombosis, including sepsis, malignancy, and disseminated intravascular coagulation.42 Because TF is the principal ligand for FVIIa, it remains a possibility that low plasma FVIIa levels are due to accelerated clearance as a result of binding to an increased number of available TF sites, whether they be in an intravascular or extravascular location. Ultimately, the possibility of accelerated clearance of FVIIa can probably only be adequately addressed by in vivo studies of the fate of labeled FVIIa in patients with high TF expression, such as SCD or sepsis.
In summary, our data unequivocally demonstrate that circulating levels of TF PCA are elevated in patients with SCD. This pathologic expression of TF may provide the proximate stimulus responsible for activation of the coagulation system, which in turn may play an important role in many of the vaso-occlusive complications of SCD.
ACKNOWLEDGMENT
The authors thank Dr James Morrissey for the gift of the soluble recombinant TF mutant, Dr Robert Hebbel for helpful discussions, and Dr Arkadiusz Dudek for assistance with FACS analysis. In addition, the assistance of Jeanne Harkness, RN, in procuring patient samples is gratefully acknowledged.
Supported in part by grants from the Minnesota Medical Foundation, by grants from the American Heart Association (Minnesota Affiliate), by National Institutes of Health Grants No. HL55219 (to N.S.K.) and HL55213 (to F.A.K.), and by grants from the Department of Veterans Affairs (R.R.B.).
Address reprint requests to Nigel S. Key, MB, MRCP, University of Minnesota Medical School, Box 480 Mayo, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: keyxx001@tc.umn.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 4. Plasma [FVIIa] is decreased in patients with SCD. Citrated plasma samples from normal controls or patients with SCD were assayed for plasma [FVIIa] as described in Patients and Methods. Each symbol represents a single data point; samples are included from both Black (•) and Caucasian (○) control subjects. Patient samples include those drawn during steady-state disease (▵) as well as during pain crisis (▴).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4216/4/m_blod41115004x.jpeg?Expires=1763489849&Signature=ZY7UdYt-6HjG4XC4WLtdsWDzl-vmv7dtw~CuFGB7pnyoqk4hlFUqRIvDWbuUChdyzntD1Mm91IXwhUgdqTvtCbcztNsQW~Dj2CoiaXpPu2PbfUN3fbm1lWvurPi8DDZF2Kk9kVic3yZfdFBvUz~01yoFkpvJAhOx6D6cE1z1hqkUUJhYxH8bTwBQn3dBgGUXh8tMTweU9VsMX9zUGNi2edT7dJYlSuHqHTL3sDtlKYU~OT9mIb3NLWxwCkDXDP9-M~06R5Pf-Gc4E4AlatR2VnxLTOWn213mXhy-k30TtKL~k4AxO~Tmo3YemTmcQp6qTeEtGOkyNV3jVW9ZuK8IGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)