To the Editor:
In the last few years a lot of reports dealt with pharmacokinetic interactions leading to elevated cyclosporine (CsA) blood concentrations. These interactions involve various drugs and food constituents, including antibiotics, antifungal agents, calcium antagonists, dietary lipids, and grapefruit juice.1,2Grapefruit juice has been shown to significantly inhibit the gut wall cytochrome P 450 isoenzyme CYP 3A4, which is important in metabolism of CsA.3
The effect appears to be caused by the components of grapefruit juice, naringin, naringenin, or other flavonoids.4,5 The bioavailability of conventional CsA is low, with variable adsorption, clearance, and distribution; dosing, safe and effective use, and toxicity of the drug are still a matter of debate even though a better bioavailability has been observed with a new CsA microemulsion formulation.6 Recently, a study was presented7on healthy adult volunteers, showing that coadministration of a defined dose of grapefruit juice could increase blood CsA concentration. A similar effect was observed in renal transplant recipients8and also in patients with autoimmune rheumatologic diseases.9 However, it is still uncertain whether the effect of grapefruit juice may be sustained over time and whether it may contribute to maximize efficacy and minimize toxicity of CsA. So, we consider it useful to report on the satisfactory effect of grapefruit juice on blood CsA concentration and clinical outcome in four resistant, CsA-dependent, hematological patients followed for 9 months.
Four patients (age 20 to 69 years) with hematological immune disorders were treated: a female with autoimmune hemolytic anemia (AIHA), a female with idiopathic throbocytopenic purpura (ITP), a male with AIHA in chronic lymphocytic leukemia (CLL), and a male with severe aplastic anemia (AA). With the exception of the AA patient, the other three patients were resistant to other immunosuppressive treatments and splenectomy, and the hematological remission was maintained with chronic administration of CsA for 13 to 58 months. Further details on these patients have been reported elsewhere.10 The AA patient was on CsA with water treatment for 3 months. Four patients (two AIHA women, one ITP woman, one AA man; age 28 to 62 years) having CsA only with water were followed for the same time as controls.
After informed consent, the grapefruit juice (250 mL) was coadministered orally with cyclosporine A capsules, outside the clinic. The drug daily dose (3 to 4 mg/kg to maintain the blood therapeutic values of 200 to 400 ng/mL) was administered simultaneously with the juice, half in the morning and half in the evening. Two patients (with AIHA and AA) used commercially available grapefruit juice and the others (with ITP and AIHA + CLL) a juice obtained by a commercially available self-squeezed grapefruit.
The CsA blood concentration was monitored every 2 weeks by Sandimmum-kit radioimmunoassay as well as blood pressure, glucose, serum creatinine, serum glutamic oxaloacetic, and pyruvic transaminase and lactate dehydrogenase levels. In all patients treated with CsA plus grapefruit juice, after 2 weeks of treatment we noted a progressive increase of CsA blood concentration and an increase of hemoglobin (Hb) and platelet levels; we also noted an increase in the white blood cell levels in the AA patient.
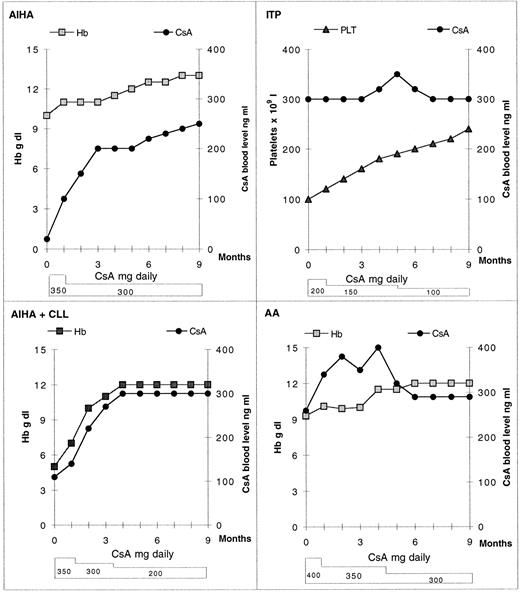
This allowed a reduction of CsA doses, especially in ITP patient (Fig 1). For all patients, the clinical outcome progressively improved (Fig 1). Neither nephrotoxicity nor other side effects were observed. The ITP patient, with drug requiring, CsA-dependent hypertension was also able to withdraw the antihypertensive drugs. In the control patients treated with CsA with water, the hematological remission was maintained over time with full CsA doses and without any possibility of CsA dose reduction.
Effect of coadministration of grapefruit juice and CsA on Hb and platelet (Plt) levels in four patients. CsA blood normal therapeutic values = 200 to 400 ng/mL.
Effect of coadministration of grapefruit juice and CsA on Hb and platelet (Plt) levels in four patients. CsA blood normal therapeutic values = 200 to 400 ng/mL.
Our data suggest that coadministration of CsA with grapefruit juice in drug-dependent hematological patients could increase CsA blood concentration and that the effect is sustained over time. This interaction, if unmonitored, is potentially dangerous, increasing toxicity of CsA. On the other hand it allows the reduction of daily required dose of drug, improving the clinical outcome on the whole with stable results and without side effects, at least in patients with hematological disorders. Moreover, the reduction of drug dosage can cut its side effects, as in one of our patients, as well as the cost of treatment.
We think that it would seem reasonable to warn the hematologists, the patients under long-term CsA treatment, and the pharmacists of this food-drug interaction.