To the Editor:
Although variables relating to disease biology and patient characteristics are strong determinants of outcome after bone marrow transplantation (BMT), the identification of these nonmodifiable factors is often of limited practical use.1 On the other hand, treatment- and transplant-related factors, which are modifiable, can potentially be manipulated in clinical practice to improve the results of transplantation.1 The number of nucleated cells infused during transplantation is a variable which is usually controllable. Therefore, it is encouraging to see that increasing this number as much as possible improves survival by reducing transplant-related mortality in patients allografted from unrelated donors.2
The effect of cell dose on disease-free survival and transplant-related mortality in 85 AML patients allografted in first remission (cell numbers not available for two patients). Higher cell doses are significantly better.
The effect of cell dose on disease-free survival and transplant-related mortality in 85 AML patients allografted in first remission (cell numbers not available for two patients). Higher cell doses are significantly better.
We have also found in a number of different studies that the nucleated cell dose significantly affects transplant-related mortality,1,3-5 survival,1,3 and the speed as well as completeness of hematologic reconstitution after autologous1,6 and allogeneic3-5 transplantation for hematologic malignancies.
Among 74 patients with acute myeloid leukemia (AML) autografted in first remission after melphalan and single-fraction total-body irradiation (TBI),1 7 of 21 patients receiving ≤2 × 108 nucleated cells/kg body weight and 7 of 53 patients receiving >2 × 108 nucleated cells/kg body weight died of treatment-related toxicity (P = .047, χ2 test). In multivariate analysis, patients receiving the higher cell dose had a significantly better disease-free survival (relative risk 2.17, P = .045).1 The higher toxic death rate and poorer disease-free survival in patients receiving ≤2 × 108 nucleated cells/kg was only partially due to incomplete or delayed hematopoietic reconstitution with resultant increase in bleeding or infections because the cell dose did not affect the probability or rapidity of engraftment significantly in this group of 74 patients.1 Because 2 × 108/kg cells is our usual target for collection during an autologous marrow harvest, those receiving ≤2 × 108/kg nucleated cells may have represented a group of patients with compromised marrow reserve due to either high-risk disease or previous chemotherapy who subsequently had problems during the transplant.
However, in a study of factors affecting hematologic recovery after unpurged autologous blood or marrow transplantation in 240 patients with acute leukemia (which included the previous 74 patients1 ),6 a nucleated cell dose of <2 × 108/kg was independently associated with slower recovery to 0.5 × 109/L neutrophils, and with slower and incomplete recovery to 50 × 109/L platelets. There was a strong correlation between neutrophil recovery and five increasing nucleated cell dose levels (<1.5, 1.5-2, >2-2.5, >2.5-3.5, and >3.5 × 108/kg).
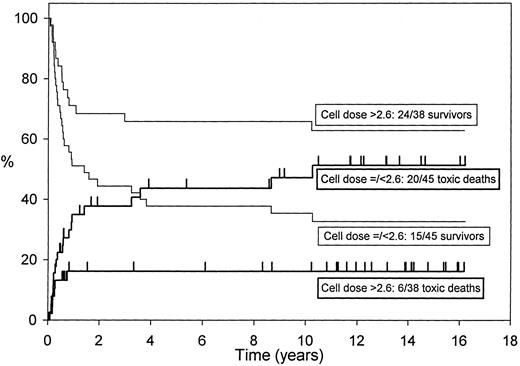
Long-term follow-up of 85 first-remission AML patients allografted from HLA-identical siblings after cyclophosphamide and single-fraction TBI with cyclosporine for graft-versus-host disease (GVHD) prophylaxis showed that infusion of a lower nucleated cell dose (≤2.6 × 108/kg) was independently predictive of increased transplant-related mortality (relative risk 2.69, P = .002).3 The higher cell dose was associated with better disease-free survival (relative risk 2.01, P = .045) in multivariate analysis.3 Figure 1 shows the effect of the cell dose in this group of patients.
Similarly, in a study of transplant-related mortality in 138 acute leukemia patients allografted after melphalan and TBI with cyclosporine ± methotrexate,4 a high infused marrow cell dose (≥2.5 × 108 total nucleated cells/kg or ≥0.6 × 108 mononuclear cells/kg) protected against fatal interstitial pneumonitis compared with lower cell doses (risk ratio 0.47, P = .023).
After allogeneic transplantation in 712 patients with hematologic malignancies, a low total leukocyte count in the third week was found to be the most significant predictor of death due to infections, hemorrhage, or graft failure within the first 3 months.7 A low nucleated cell dose (<2.5 × 108/kg) was independently associated with increased risk of death due to these causes (relative risk 2.2, P = .025).7
Because of their impact on transplant-related mortality, the number of infused cells could potentially affect conclusions of important transplant studies. For example, two large, recently concluded randomized studies (United Kingdom MRC AML 10,8 and the European Organization for Research and Treatment of Cancer [EORTC] and Gruppo Italiano Malattie Ematologiche Maligne Dell'Adulto [GIMEMA] cooperative group9 ) which showed no advantage for autologous BMT (ABMT) in first remission AML, had recommended 1 × 108 nucleated cells/kg as the target collection for ABMT. It is possible that inadequate cell doses may have compromised engraftment and increased mortality in the ABMT arm in these studies, negating any beneficial effect ABMT may have had. However, details on the effect of cell doses are not available for either of the two studies.8,9 The late survival advantage that appears to have emerged in the MRC AML 10 study for the ABMT arm10 may well have emerged earlier if the cell doses had been better controlled.
Using peripheral blood stem cells may be a better way of obtaining faster and more complete engraftment in patients autografted for AML,6,11 but may be associated with a higher relapse rate.12,13 The high relapse rate may be a result of reinfusion of more residual disease secondary to infusion of nucleated cell numbers that are much larger than those infused during marrow transplantation.12 Because of these concerns, we do not use blood-derived stem cells for autografting patients with AML, but aim for a target collection of 2.5 to 3 × 108 nucleated marrow cells/kg after two courses of consolidation chemotherapy.1
After appreciating the importance of cell dose, in order to at least partially eliminate this issue from a double-blind randomized comparison of peripheral blood versus marrow for allogeneic transplantation which has just finished recruitment,14 we chose 3 × 108 nucleated donor marrow cells per kilogram patient weight as the target for collection. Despite this, and the fact that all the marrow donors underwent just one apheresis procedure, the difference between marrow and blood-derived nucleated and CD34+ cell numbers remained highly significant (Table 1).14
A more precise measure of the progenitor cell content of a hematopoietic stem cell graft is the number of CD34+ cells. Mavroudis et al15 showed that a low CD34+ cell dose was associated with increased mortality after T-cell–depleted allogeneic BMT. The only problem with using CD34+ cell numbers is that this may not measure the contribution of accessory cells including lymphocytes to engraftment and immune recovery.
Until more definitive data are available on the effect of CD34+ cell numbers on transplant-related mortality, it would be reasonable to aim for 2.5 to 3 × 108 nucleated marrow cells for autografting in acute leukemia, at least 3 × 108 nucleated marrow cells for allogeneic transplantation from HLA-identical siblings, and at least 4 × 108 nucleated marrow cells for allogeneic transplantation from unrelated donors.