Abstract
Loss of physical performance is a universal problem of cancer patients undergoing chemotherapy. We postulated that this impairment can be partially prevented by aerobic exercise. In a randomized study, 33 cancer patients receiving high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation (training group, T) performed an exercise program consisting of biking on an ergometer in the supine position after an interval-training pattern for 30 minutes daily during hospitalization. Patients in the control group (C, n = 37) did not train. Maximal physical performance was assessed with a treadmill test by admission and discharge. Physical performance of the two groups was not different on admission. The decrement in performance during hospitalization was 27% greater in the control group than in the training group (P = .05); this resulted in a significantly higher maximal physical performance at discharge in the trained patients (P = .04). Duration of neutropenia (P = .01) and thrombopenia (P = .06), severity of diarrhea (P = .04), severity of pain (P = .01), and duration of hospitalization (P = .03) were reduced in the training group. We conclude that aerobic exercise can be safely carried out immediately after high-dose chemotherapy and can partially prevent loss of physical performance. Based on the potential significance of the observed outcomes, further studies are warranted to confirm our results.
FATIGUE AND IMPAIRMENT of physical performance are common, and sometimes serious, side effects of cancer treatment. It has been estimated that the problem affects up to 70% of cancer patients during chemotherapy or radiotherapy.1-4 For many patients, particularly in the recovery phase immediately after treatment, low physical performance imposes limitations on basic daily activities. Postulated etiologic mechanisms for the development of this problem include impaired nutritional status, sleep disturbances, biochemical changes secondary to disease and treatment, psychosocial factors, and reduced level of activity.5 However, the causes of impaired physical performance in this setting are not yet fully understood. One frequently underestimated factor contributing to loss of physical performance in cancer patients is the lack of muscular activity during in-hospital treatment. Inactivity inevitably results in muscular catabolism, producing rapid loss of performance. The deleterious effects of prolonged bedrest are well documented.6-8
Aerobic exercise (defined as the rhythmical contraction and relaxation of large muscle masses over an extended time) has been shown to improve physical performance and reduce fatigue in cancer patients.9-12 However, this is not yet a widely accepted concept. Furthermore, some physicians fear that vigorous exertion may be harmful for cancer patients, although no literature reports support this notion.
We investigated the effects of aerobic exercise on the loss of physical performance and on the incidence and severity of complications in patients undergoing high-dose chemotherapy (HDC) followed by autologous peripheral blood stem cell transplantation (PBSCT).
MATERIALS AND METHODS
Eighty patients with solid tumors selected for treatment with HDC were considered for participation in the study. Inclusion criteria were the following: malignancy confirmed by biopsy; Eastern Cooperative Oncology Group (ECOG) performance score of 0-2; age between 18 and 60 years; no evidence of impairment of cardiac, pulmonary, renal, and hepatic function; absence of bony metastases in the lower extremities; and transplantation of at least 1 × 106 CD34+ peripheral blood stem cells/kg body weight. The study was approved by the Ethics Committee of the University of Freiburg and informed consent was obtained from all patients. From the 80 potential participants, 72 (90%) fulfilled inclusion criteria and were enrolled in the study.
Before HDC, all patients received one to four chemotherapy cycles consisting of etoposide 500 mg/m2, ifosfamide 4 g/m2, cisplatinum 50 mg/m2, with or without epirubicine 50 mg/m2 (VIP/VIP-E), followed by administration of granulocyte colony-stimulating factor (G-CSF ) at a dose of 5 μg/kg body weight/d. Ten days after the last cycle of chemotherapy, leukapheresis for collection of peripheral blood stem cells was performed. In the week before admission for HDC, all patients underwent a complete physical and cardiovascular examination (electrocardiogram [ECG] and echocardiogram). Two patients showed abnormalities on electrocardiography and were excluded from the study.
Overall, 70 patients fulfilled all requirements for participation (Table 1). On their first day of hospitalization, patients were assigned randomly to a training (T) or a control group (C). In the week preceding HDC, a treadmill stress-test under continuous ECG monitoring (starting at 3 km/h and 1.5% elevation, acceleration of 1 km/h every third minute by unchanged elevation and continued until exhaustion) was performed for assessment of physical performance. This stress-test is one of the standard protocols used in Germany for assessment of maximal physical performance of patients with reduced physical performance as sequel of chronic diseases and correlates highly with VO2max.13 14 During the test patients were not allowed to hold handrails.
All patients underwent high-dose chemotherapy with cumulative doses of etoposide 1.5 g/m2, ifosfamide 12 g/m2, and carboplatin 750-1500 mg/m2 (VIC, n = 57); 13 patients also received epirubicine, 150 mg/m2 (VIC-E, n = 13). One patient in the training group refused to complete chemotherapy and received the full dose of etoposide and only 2/3 of the planed dose of ifosfamide and carboplatin. After chemotherapy all patients received autologous PBSCT and daily subcutaneous (sc) injections of G-CSF at the above-mentioned dose during neutropenia (absolute neutrophil count [ANC] <0.5 × 109/L).
Patients in the training group performed a daily program of aerobic exercise, consisting of “biking” with a bed ergometer (Rotomed; Reck Machinenbau GmbH, Betzenweiler, Germany). This device allows one to simulate a biking motion without leaving bed. The patients “biked” for 1 minute with an intensity sufficient to reach a heart rate equivalent to at least 50% of the cardiac reserve, calculated as 220 minus age minus rest heart rate.14 The procedure was repeated 15 times with pauses of 1 minute between bouts; therefore, training was carried out for a total of 30 minutes each day. Mean workload during the training program was 32 ± 5 W (range, 20 to 40 W). During training sessions patients were continuously supervised by instructed study personnel. Physical performance of patients changed during hospitalization as a result of variations in clinical parameters (eg, hemoglobin concentration, nutritional status, infections, etc). Therefore, pedaling speed was re-adjusted daily to achieve the aimed heart rate and then kept constant within single training sessions (15 bouts); the daily pedaling frequency varied between 30 and 50 cycles/min. To calculate the mean heart rate during a training session, heart rate was assessed at the end of each 1-minute workload and added; heart rate during pauses was not considered.
Patients with fever (>37.5°C) or platelet counts below 10 × 109/L were instructed to interrupt training. Patients with World Health Organization (WHO) grade I and II infections and no other complications restarted training after abatement of fever and performed the training until discharge, whereas patients with severe infections (WHO grade III) or multiple complications were instructed not to resume training. Two patients in the training group elected to abandon the study after the second training unit for personal reasons; these patients did not develop major complications during the rest of hospitalization.
Complete blood counts and serum chemistry (including evaluation of hepatic and renal function) were carried out daily between 6:00 and 8:00 AM, ie, after at least 12 hours without training. Criterion for blood transfusion was hemoglobin concentration of less than 8 g/dL; criteria for platelet transfusion were thrombopenia < 20 × 109/L or bleeding. Discharge criteria were trilinear hematopoietic reconstitution, transfusion independency, an afebrile period of at least 2 days after discontinuing intravenous antibiotics, resolution of mucositis, ability to tolerate solid food, and absence of clinical signs of fluid overload or dehydration.
On day of discharge, a second cardiologic examination consisting of an ECG, echocardiogram, and stress-test was carried out. By this time most patients were still recovering from chemotherapy-related mucositis and could not tolerate a mouthpiece for direct assessment of VO2max. Therefore, this parameter was not assessed. Instead, maximal physical performance was defined as the maximal speed (in kilometers per hour) reached in the treadmill stress-test. Because VO2max is a function of maximal walking speed,14 these two parameters are intimately correlated.
Four patients in the control group (three patients refusing participation, another patient presenting with atrial fibrillation) and two patients in the training group (refusal of participation) did not undergo a second stress-test. Severity of chemotherapy-related complications of these six patients was included in the statistical analysis; however, data of their physical performance were not considered.
Hematologic and nonhematologic toxicity were analyzed according to standard WHO criteria.15 These assessments were made by an investigator who was blinded to the patients' assignments to control and treatment group. Duration of hospitalization was measured as number of days between blood stem cells reinfusion and discharge. Based on the characteristics of the study, we considered a blinding of the medical team difficult to warrant. Therefore, to avoid an intentional or unintentional manipulation of results, medical teams were not informed about the secondary endpoints of the study.
Statistical analysis. The study was performed following the “intention-to-treat” principle. Primary endpoint was the loss of maximal physical performance (defined as maximal speed in kilometers per hour on a treadmill test) during hospital stay; a difference in the loss of physical performance of at least 25% between the two groups was considered to be clinically relevant. To detect this difference with a probability of an α and β error of 5% and 10%, respectively, at least 30 patients in each group were required.
Nominal data were compared with the Fisher's exact test, normally distributed continuous data with the Student's t-test, and categorical and not normally distributed continuous data with the Mann-Whitney U test; all tests were two-tailed. To rule out the effect of confounding factors on the observed outcomes, we performed a post-hoc backward multiple regression analysis including secondary endpoints found to differ significantly between the groups as dependent variables, and age, body mass index, maximal physical performance (in kilometers per hour) by admission, total dose of carboplatin, number of peripheral blood stem cells retransfused, and training as independent variables. All calculations were performed with SPSS for Windows 6.1.2 (SPSS GmbH Software, Munich, Germany). A value of P < .05 was accepted as statistically significant. Data are expressed as mean ± standard deviation. Because many of the evaluated parameters considered in the statistical analysis were clearly related to a single event (hematopoietic reconstitution), Bonferroni corrections were not applied.
RESULTS
At the beginning of the study no differences in the baseline characteristics of the two groups were observed (Table 1). Patients in the training group performed the aerobic exercise program for 82% (±10%) of hospital days.
Physical performance. Maximum performance of both groups was not different at initiation of the study (see Table 2). Loss of performance during hospitalization was 27% higher in the control group than in the training group (absolute values: training group 14%, control group 19%, P = .05, resulting in a significant difference between the maximal performance of both groups at discharge (P = .04).
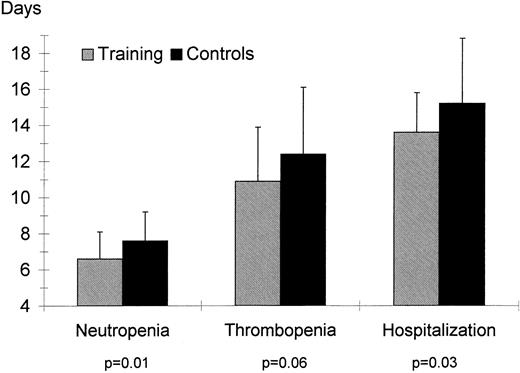
Duration of neutropenia (ANC < 0.5 × 109/L), thrombopenia (platelets <50 × 109/L), and hospitalization (calculated as days between stem cell reinfusion and discharge) for the training and control groups.
Duration of neutropenia (ANC < 0.5 × 109/L), thrombopenia (platelets <50 × 109/L), and hospitalization (calculated as days between stem cell reinfusion and discharge) for the training and control groups.
Hematological indexes. Hemoglobin concentration and hematocrit were not different between the groups at admission or discharge. The training group had a shorter duration of neutropenia (ANC < 5 × 109/L, P = .01) and of thrombopenia (platelets <50 × 109, P = .06, see Fig 1); the requirement for platelet transfusions was also lower in the training group (P = .06). Number of erythrocytes transfusions was not different for the two groups (P = .92).
Cardiologic examinations. One patient in the control group developed atrial fibrillation shortly after HDC. For the remaining patients, ECG at rest and during stress-test, and echocardiographic assessment of cardiac dimensions and function (heart volume, HV and shortening fraction, SF ) showed no changes between the two examinations (by admission: T: HV 733 ± 134 mL, SF 37% ± 4%, C: HV 733 ± 140 mL, SF 38% ± 5%; at discharge: T: HV 713 ± 128 mL, SF 36% ± 4%; C: HV 720 ± 143 mL, SF 37% ± 6%).
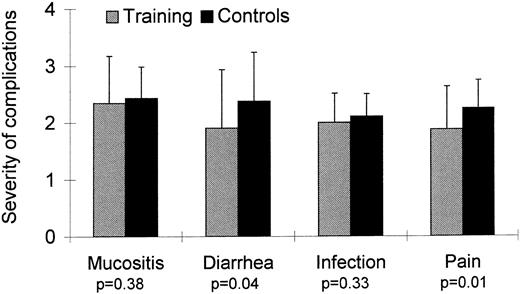
Toxicity of HDC. Fifteen patients (21%) developed significant complications: severe infection (4 patients in the training group, 5 controls), moderate infection combined with diarrhea (1 patient in each group), hepatic hemorrhage (1 patient in the training group), atrial fibrillation (1 patient in the control group), chemotherapy-related seizures (1 control), and moderate infection followed by allergic reaction after platelet transfusion (1 patient in the training group). Severity of mucositis was not different for the two groups (Fig 2). The incidence of diarrhea was lower in the training group (P = .04). Severity of pain was for patients in the training group lower than for control patients (P = .01). No patient in the training or control group developed signs of renal toxicity. One patient in the training group died of hepatic hemorrhage on the fourth day after PBSCT. Severe intraabdominal bleeding necessitated laparotomy, which showed liver necrosis. This complication is a well-known serious side-effect of treatment with alkylating agents and has been described with high-dose cyclophosphamide, etoposide, and carboplatin. Bleeding began suddenly 8 hours after the end of the last workout; the patient was physically active without symptoms between conclusion of training and onset of bleeding. In light of these considerations, a causal relationship between this complication and training seems extremely unlikely. No other patient in the training or control group developed signs of hepatic toxicity.
Duration of hospitalization. Duration of hospitalization was shorter for patients in the training group than for patients in the control group (P = .03, Fig 1).
Multiple regression analysis showed aerobic training to be the most significant predictor of loss of physical performance, duration of neutropenia and thrombopenia, number of platelets transfusions, severity of pain and diarrhea, and duration of hospitalization (Table 3).
DISCUSSION
Loss of physical performance and fatigue are a universal phenomenon in cancer patients after myelotoxic chemotherapy. After discharge, most patients find it difficult to perform daily activities. Moreover, some patients may need weeks or months to regain their pretreatment level of fitness.2 Our study shows that the severe loss of performance regularly observed after HDC can be at least partially prevented with adequate rehabilitative measures. Our results also indicate that starting rehabilitation immediately after completion of HDC is possible without increasing morbidity.
Moreover, several positive outcomes were observed in patients who performed aerobic training during hospitalization. Firstly, duration of neutropenia in the training group was significantly shorter than for controls. Because neutropenic infections represent one of the principal complications after HDC and PBSCT, the importance of this observation is obvious. Indeed, 4 patients in the training group (13%) but only 1 patient in the control group (3%) developed a light infection, whereas the remaining patients developed moderate to severe infections. Although many effects of physical activity on the immune system have been described,16 the exact mechanism underlying these effects remains to be determined. Further investigation seems warranted to explain the interactions between physical activity and hematopoiesis indicated by our study. Whether accelerated intestinal epithelial cell reconstitution, in addition to improved immune function, may lead to the lower severity of diarrhea observed in the training group must also remain speculative at this time.
Another positive effect observed was the different need for analgesics for the two groups. Eight patients in the training group (25%) but only 1 patient in the control group (3%) did not need analgesics during hospitalization; moreover, a substantially higher percentage of patients in the control group required treatment with opioid analgesics (training group 4 patients, 12%; control group 10 patients, 27%). Literature reports offer an explanation for this finding. In several studies, physical exercise has been shown to elevate pain threshold17-19; the mechanisms proposed to underlie this effect are an activation of central pain inhibitory systems and a higher production of endorphins.20
Average duration of hospitalization was shorter for the training group than for controls. Because the decision to discharge a patient is made by the medical team based on clinical considerations, we cannot exclude that subjective factors affected this outcome. However, several objective parameters that also influence duration of hospitalization showed differences between the training and control groups. Furthermore, the medical staff was not informed about the secondary endpoints of the study. Therefore, the shorter duration of hospitalization for the training group may not entirely be an artifact because of subjective decisions made by the medical staff.
A critical point in our study was the comparability of effort of patients in the training and control groups during both stress-tests. To analyze this point, we compared the percentage of maximal predicted heart rate (220 minus age) reached by participants in the study in the stress-tests. No differences were found between the training and control groups (Table 2). These results indicate a comparable degree of effort in the two groups by all tests. Moreover, mean heart rate in all tests was more than 90% of the maximal predicted heart rate, indicating that the tests were performed until exhaustion and were not prematurely interrupted due to factors like coordinatory problems or pain.14
Echocardiography, resting, and exercise ECG showed no pathological changes in the training group at final testing. Furthermore, no patient in the training group developed clinical signs of cardiotoxicity during the 2 months after chemotherapy. This indicates that patients with no signs of impaired cardiac function can perform aerobic exercise after HDC with the described protocol and autologous PBSCT without fear of cardiac complications.
In the present study we have furnished evidence that aerobic exercise may be useful in preventing the loss of physical performance in cancer patients after myelotoxic chemotherapy. Furthermore, these data, while limited, suggest that exercise can be performed safely after HDC and PBSCT. Likewise, our finding of reduced chemotherapy-related complications in trained subjects is provocative. Clearly, all of these findings require confirmation. A randomized prospective study including patients with hematologic malignancies undergoing autologous and allogeneic bone marrow transplantation has been initiated to address this question.
ACKNOWLEDGMENT
The authors thank the doctoral students Annette Hahn, Ulrike Augustin, Murad Ruf, and Christoph Janzen, who supervised the patients during the study; Dominik Grathwohl for his advice concerning statistics; and Monika Tilmann, MD, and Ralf Beneke, MD, for revision of the manuscript and many helpful suggestions.
Supported by the Nenad Keul Foundation Preventive Medicine, Freiburg in Breisgau, Germany.
Address reprint requests to Fernando Dimeo, MD, Free University Berlin, University Medical Center Benjamin Franklin, Department of Sports Medicine, Clayallee 229, 14195 Berlin, Germany.