Abstract
A gene causing Dyskeratosis Congenita (DC), a rare genetic disorder associated with bone marrow failure, has been mapped to chromosome Xq28, but autosomal inheritance of the disease has also been reported. We have investigated the pattern of X-inactivation in the peripheral blood of carriers of DC using the methylation-sensitive Hpa II site in the androgen receptor gene (HUMARA). In 5 different families in which the inheritance of DC appears to be X-linked, all 16 carriers showed skewed X-inactivation patterns. These cases indicate that, in the hematopoiesis of heterozygous females, cells expressing the normal DC allele have a growth advantage over cells that express the mutant allele. In 7 other families with sporadic cases of DC or with an uncertain pattern of inheritance, both skewed and normal patterns of X-inactivation were observed. In these families or where crucial family members are unavailable, the study of X-inactivation patterns will add to linkage analysis in providing information about carrier status.
DYSKERATOSIS CONGENITA (DC) is a rare inherited disorder associated with bone marrow failure and a predisposition to malignancy. Although it is not always easy to make the diagnosis of DC, the disease is characterized by nail dystrophy, mucosal leukoplakia, and reticulate skin pigmentation.1-3 DC is often inherited as an X-linked recessive disorder (DC, MIM 305000), and in one large pedigree, Connor et al4 mapped the DC gene to Xq28. This linkage has been confirmed in three additional families,5 and we have recently mapped the DC locus to 3.8 Mb of DNA within this region,6 although the gene itself has not yet been identified.
There is also evidence that the disease is heterogeneous, and autosomal dominant and recessive modes of inheritance have been recognized (MIM 127550 and MIM 22430). Although we have studied a number of multiplex families in which the disease is present, 16 of the 29 families that have been referred to us are sporadic, with only 1 family member affected. Although most of these cases are male and therefore consistent with an X-linked mode of inheritance, genetic counselling of family members is difficult in view of the heterogeneity of this disorder.
Skewed X-inactivation has been observed in X-linked disorders such as X-linked agammaglobulinemia,7 incontinentia pigmenti type 2,8 and Wiscott-Aldrich syndrome, in which skewing has been used as a method for carrier detection.9 In this study, we show that skewed X-inactivation is also seen in female carriers of DC in 5 different families in which the disease is clearly inherited as an X-linked disorder, consistent with a previous report on 1 X-linked family and the mother of a single sporadic case.10 We have also extended the analysis to our unique collection of sporadic families with DC to see if this is a consistent finding and to see if X-inactivation analysis can help us to recognize the X-linked form of the disease and to determine the carrier status of female family members.
MATERIALS AND METHODS
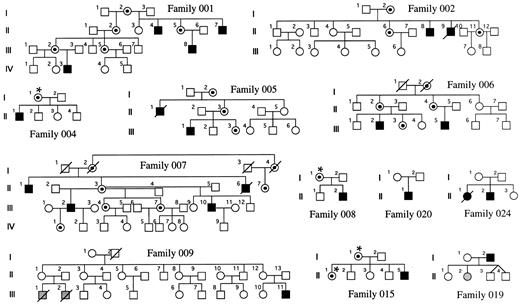
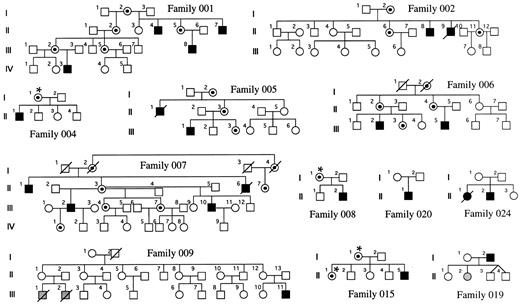
Families and sample preparation.Families with DC were obtained through the DC registry established at the Hammersmith Hospital. In each family, the diagnosis of DC was made when the index case had the three following muco-cutaneous features by the age of 15 years: (1) skin pigmentation, (2) nail dystrophy, and (3) mucosal leukoplakia. Twelve families could be included in this study because fresh blood samples had been referred from female relatives of the index case, and these females were informative for the HUMARA polymorphism (see below). They are shown in Fig 1. Five of these families have been previously described,3-5 and in these, linkage of the disease to markers in Xq28 has been established (families 001, 002, 005, 006, and 007). In 4 of the remaining 7 families, there is only a single affected male known to us (families 004, 008, 015, and 020). In 1 family (019), the father is affected and one of his daughters has aplastic anemia, but no other features of DC. In another family (024), there is an affected male and an affected female in one generation. In the remaining family, which has been described11 (family 009), there is one boy who is clearly affected with DC and he has two male first cousins who died very young from bone marrow failure. For the 25 relevant females, DNA was obtained from a whole blood sample by conventional phenol-chloroform extraction.12
DC families. The carrier status of women marked with an asterisk has been inferred from their X-inactivation status. The shaded individuals have aplasia but do not show the dermatologic features of DC.
DC families. The carrier status of women marked with an asterisk has been inferred from their X-inactivation status. The shaded individuals have aplasia but do not show the dermatologic features of DC.
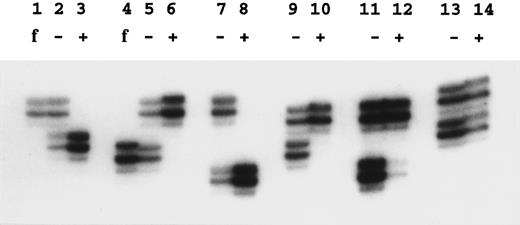
Analysis of X-inactivation.X-inactivation was analyzed using the methylation-sensitive Hpa II site located near the highly polymorphic trinucleotide repeat in the human androgen receptor gene (HUMARA)13 in Xq11.2-Xq12. Amplification was performed after or without Hpa II digestion with primers flanking the CAG repeat and the Hpa II site. After Hpa II digestion, no amplification product is seen from the active X-chromosome, because the site is unmethylated and cleaved. Amplification products were analyzed on standard 6% sequencing polyacrylamide gels containing 8 mol/L urea. The only modification we have made to the previously described method13 was a reduction of the number of polymerase chain reaction cycles from 28 to 25. The dilution of amplified DNA in formamide loading dye was sometimes adjusted to even out the DNA load. Quantification of autradiographs was performed at short exposure times (< 16 hours) using a UVP camera system (GDS7600; Ultra Violet Products Ltd, Cambridge, UK) and the NIH Image/68k 1.57 program (Wayne Rasband [wayne@helix.nih.gov], National Institutes of Health, Bethesda, MD).
RESULTS
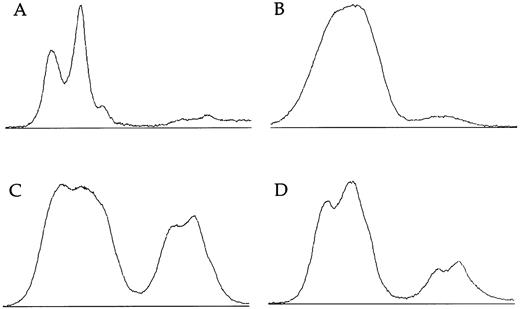
X-linked families.In the 5 families in which we have shown linkage of the DC gene to markers in Xq28, all of the 16 female carriers analyzed show skewed X-inactivation patterns (Table 1 and Fig 2). This is true for both the obligate carriers as well as for those that we predict to be carriers based on linkage analysis. In 2 of these females (family 005, III:3 and family 006, II:4), the skewing is not complete, and a faint residual band can be seen for one allele (eg, Fig 2, lane 12). From the densitometry plots (Fig 3A and B), we estimate that the minor band in these cases represents 5.5% of the total signal in the obligate carrier (006, II:4) and 3% in the predicted carrier (005, III:3).
Examples of X-inactivation analysis. Amplification by polymerase chain reaction across the HUMARA polymorphism was performed as described in the Materials and Methods. Lanes marked f show the allele amplified from the father of the female studied in the next two lanes; − is without Hpa II digestion and + is with Hpa II digestion before amplification. Lanes 2, 3, and 5 through 12 show females from X-linked families. In these samples note the skewed inactivation patterns, although lane 12 is incompletely skewed. Lanes 13 and 14 show a normal pattern in the mother of family 024.
Examples of X-inactivation analysis. Amplification by polymerase chain reaction across the HUMARA polymorphism was performed as described in the Materials and Methods. Lanes marked f show the allele amplified from the father of the female studied in the next two lanes; − is without Hpa II digestion and + is with Hpa II digestion before amplification. Lanes 2, 3, and 5 through 12 show females from X-linked families. In these samples note the skewed inactivation patterns, although lane 12 is incompletely skewed. Lanes 13 and 14 show a normal pattern in the mother of family 024.
Densitometry plots of X-inactivation analysis. Individual lanes showing amplification of the HUMARA gene after Hpa II digestion are plotted using the NIH image program. Autoradiographs were exposed for less than 16 hours. (A) The incompletely skewed female shown in lane 12 of Fig 2 (family 6, II:4). (B) The other incompletely skewed female from an X-linked family (family 005, III:3). (C) The mother of an affected boy from the family with an uncertain pattern of inheritance (family 009, II:12). (D) Her sister (family 009, II:1).
Densitometry plots of X-inactivation analysis. Individual lanes showing amplification of the HUMARA gene after Hpa II digestion are plotted using the NIH image program. Autoradiographs were exposed for less than 16 hours. (A) The incompletely skewed female shown in lane 12 of Fig 2 (family 6, II:4). (B) The other incompletely skewed female from an X-linked family (family 005, III:3). (C) The mother of an affected boy from the family with an uncertain pattern of inheritance (family 009, II:12). (D) Her sister (family 009, II:1).
In 3 of these families in which we have analyzed the appropriate fathers, we can show that it is the paternal allele that fails to amplify after Hpa II digestion (eg, Fig 2, lanes 1 through 6). Therefore, it is the maternal chromosome, on which the mutated DC gene is carried, that is always methylated and inactive.
Relatives of sporadic cases.In 5 of the families investigated, there is only a single affected male. Three of the mothers of these patients (in families 004, 008, and 015) show skewed X-inactivation patterns, although in one of them (family 015) this skewing is incomplete (Table 1). A sister of a sporadic case (family 015, II:1), who is predicted to be a carrier by linkage analysis, also shows a skewed pattern. However, 1 mother (in family 020) shows a random pattern of X-inactivation. In the fifth sporadic family (019), the affected male has two daughters who would be obligate carriers if the DC was X-linked in this family: one daughter has aplastic anemia but is uninformative for the HUMARA polymorphism, but her sister (019, II:1) shows a random pattern of X-inactivation.
Familial DC, probably unlinked to Xq28.In family 024, there is an affected female and an affected male in one generation. Their mother shows a random pattern of X-inactivation (Fig 2, lanes 13 and 14). In 1 previously published family11 (009), a single boy is clearly affected with DC. He has two male cousins that died very young with bone marrow failure, and so it is proposed that these boys also had DC; the inheritance therefore appears to fit an X-linked pattern. However, in this family, the disease gene does not appear to be linked to markers in Xq28 (data not shown). Although slightly skewed, both of the relevant mothers in this family (009, II:1 and II:12) have X-inactivation patterns within the normal range:7,14 the relative intensities of the minor bands are measured at 33% (Fig 3C) and 18% (Fig 3D) in densitometry plots.
DISCUSSION
In this study we have shown that of 16 females that were obligate or predicted carriers of X-linked DC all showed skewed X-inactivation patterns. Although skewing may be observed in approximately 9% of normal subjects15 and older women in general are more likely to show a skewed pattern,16 the consistency and completeness with which it is observed in these carriers indicates that it could not have occurred by chance alone. At an early stage in development in female mammals, either the paternal or maternal X chromosome is inactivated at random in each cell. Therefore, the finding of preferential inactivation of the DC X chromosome in blood cells of female DC carriers indicates that cells expressing the normal allele at this locus have a growth or survival advantage over cells that express the mutated allele. This finding supports the idea that the product of the DC gene plays an important role in hematopoiesis. It is also consistent with our previous observation that the growth and proliferation of DC fibroblasts is abnormal.17
Two of the carriers, one of whom is a 15-year-old girl, did not show complete skewing. In affected males, the age at which symptoms of DC develop can vary considerably, presumably due to different mutations, and it is possible that this is reflected in the age and the degree to which skewed X-inactivation is manifested in female carriers. It may be appropriate to test other cells of young female carriers such as fibroblasts, because if a certain number of cell divisions are required before the putative growth advantage is manifested, fibroblasts may reach this state before lymphocytes.
A previous report has also shown highly skewed X-inactivation patterns in a single X-linked family with DC and in the mother of one sporadic case.10 The consistency of this finding that we have seen here in 5 other families with the X-linked form of DC allows us to extend the analysis to study the relatives of sporadic cases of the disease. Although the likely form of inheritance of DC in most of these families is as an X-linked recessive trait, it is possible that the inheritance is autosomal or that the index case is the product of a de novo mutation. We have found that, of 4 tested, 3 mothers of different sporadic cases of DC (families 004, 008, and 015) showed a skewed pattern of X-inactivation. We believe that this information helps us in counselling the families, allowing us to be more confident of the predictions made through linkage analysis using Xq28 markers, and even enables us to search for recombination events if there are informative meioses.
We have also seen random X-inactivation patterns in 2 mothers (families 020 and 024) and 1 daughter (family 019) of unrelated patients with DC. In these families, we can suggest that the disease is either not X-linked or that there is a de novo mutation. In 1 family (009) in which we are uncertain as to the pattern of inheritance of the disease, we find only slightly skewed patterns of X-inactivation that could be considered to fit within the normal range. If the disease is indeed X-linked in this family, then this would confound our hypothesis that the carriers of the X-linked form of DC consistently show highly skewed patterns, although it is also possible that the disease in this family is caused by another X-linked gene that is not the DC gene in Xq28, and this does not produce skewed inactivation in female carriers. It is clear that some of the suggestions made here will only be confirmed or refuted when the DC gene in Xq28 has been cloned, enabling direct mutation analysis.
On the basis of X-inactivation studies it has been suggested that a large proportion of patients with acquired aplastic anemia exhibit clonal hematopoiesis,18,19 although other studies find clonal hematopoiesis in a smaller number of such patients.20 Our results suggest that the relationship between X-inactivation and clonal hematopoiesis may not be so straightforward. Specifically, if the expression of any X-linked gene is defective in aplasia, polyclonal hematopoiesis may appear to be monoclonal, owing to a skewed X-inactivation pattern resulting from the growth or survival advantage of all cells expressing the nondefective X chromosome.
In conclusion, the studies presented here, along with other recent findings,10 now provide sufficient evidence to suggest that the X-inactivation assay can be used with some confidence to determine carrier status in potential female carriers of X-linked DC. In addition, we have shown that the assay can be useful in making a diagnosis of X-linked DC in sporadic cases by testing the mothers and/or sisters of affected individuals, enabling us to counsel these families appropriately. However, it should be borne in mind that some skewing can occur in normal females and that in young female carriers skewing may be incomplete.
ACKNOWLEDGMENT
The authors thank the members of participating families and the doctors who have referred samples to us: Prof B. Alter, Dr A. Copplestone, Dr G.L. Forni, Dr A. Fryer, Prof E. Gluckman, Prof R.E. Hall, Dr G. Lowe, Dr D. Oscier, Prof J.A. Raeburn, Dr I. Roberts, Dr J. Ross, and Dr O. Smith. The authors are also grateful for the support of Prof J.M. Goldman.
Supported by the MRC (UK) and through funds from the Department of Haematology, RPMS, London, UK.
Address reprint requests to T.J. Vulliamy, PhD, Department of Haematology, Royal Postgraduate Medical School, Hammersmith Hospital, Ducane Road, London W12 ONN, UK.