Abstract
Factor V gene polymorphisms were investigated to detect components that may contribute to the activated protein C (APC) resistance phenotype in patients with venous thromboembolism. A specific factor V gene haplotype (HR2) was defined by six polymorphisms and its frequency was found to be similar in normal subjects coming from Italy (0.08), India (0.1), and Somalia (0.08), indicating that it was originated by ancestral mutational events. The relationship between the distribution of normalized APC ratios obtained with the functional assay and haplotype frequency was analyzed in patients heterozygous for factor V R506Q (factor V Leiden). The HR2 haplotype was significantly more frequent in patients with ratios below the 15th percentile than in those with higher ratios or in normal controls. Moreover, the study of 10 patients with APC resistance in the absence of the factor V R506Q mutation showed a 50-fold higher frequency of HR2 homozygotes. The HR2 haplotype was associated with significantly lower APC ratios both in patients with venous thromboembolism and in age- and sex-matched controls. However, the two groups showed similar HR2 haplotype frequencies. Plasma mixing experiments showed that an artificially created double heterozygote for the factor V R506Q mutation and the HR2 haplotype had an APC ratio lower than that expected for a simple R506Q heterozygote. Time-course experiments evaluating the decay of factor V in plasma showed the normal stability of the molecule encoded by the factor V gene marked by the HR2 haplotype, which ruled out the presence of a pseudo-homozygous APC resistance mechanism. Our results provide new insights into the presence of factor V genetic components other than the factor V R506Q that are able to contribute to the APC resistance phenotype in patients with venous thromboembolism.
ACTIVATED PROTEIN C (APC) plays a major role1 in the control of the coagulation cascade by inactivating proteolytically both activated factor V (FVa) and activated factor VIII. A condition characterized by a poor anticoagulant response to APC (APC resistance), detectable by a plasma functional assay, has been described.2 In the Caucasian population, most cases of APC resistance, a recognized risk factor for venous thrombosis,3 are accounted for by the FV R506Q mutation (FV Leiden), which suppresses one of the APC cleavage sites on FVa.4 As a result, FVa inactivation, although not abolished, proceeds at a much slower rate.5 6
Laboratory screening of APC resistance is based on functional assays and confirmed by genetic studies that detect the FV R506Q. In approximately 5% to 10% of patients with venous thromboembolism, the APC resistance phenotype is observed in the absence of the R506Q mutation,7 which may be partly explained by the poor specificity of most of the functional coagulation-based APC resistance assays. Poor specificity, in turn, is often related to acquired conditions that influence these assays, such as elevated factor VIII levels, pregnancy, or the presence of antiphospholipid antibodies.7 However, in other cases, genetic components other than the FV R506Q mutation may produce the APC resistance phenotype or modulate its expression in FV R506Q heterozygotes. Because the R506Q mutation seems to be invariably associated with a single FV gene,8 additional genetic variation in these subjects may reside on the counterpart FV gene. An example is provided by pseudo-homozygous APC resistance. This condition,9 10 which is found in subjects doubly heterozygous for the R506Q mutation and FV deficiency, is characterized by a degree of APC resistance similar to that observed in the homozygous condition for the FV R506Q mutation.
The detection of several polymorphisms in the coding region of the FV gene, and particularly in the large exon 13,11 12 offers the opportunity to perform haplotype analysis, a powerful tool to investigate the presence of additional FV intragenic components contributing to the APC resistance phenotype. We evaluated the distribution of a peculiar FV gene haplotype in selected populations as well as its relationship with the APC resistance phenotype. Our results provide evidence that FV genetic components other than the R506Q mutation contribute to the APC resistance phenotype.
MATERIALS AND METHODS
Blood collection and storage.Venous blood was drawn in sodium citrate (12.9 mmol/L) and immediately centrifuged at 2,000g for 20 minutes at 4°C. Plasma was separated, snap-frozen, and stored in aliquots at −80°C.
Functional assay for resistance to APC.The original assay2 was used with APC at 5 nmol/L in the clotting mixture. Results were expressed as normalized ratio as follows: clotting time of the patient plasma with APC addition/clotting time of the patient plasma without APC addition divided by clotting time of the reference plasma with APC addition/clotting time of the reference plasma without APC addition. Reference plasma was a pool of 40 normal plasmas from individuals who were not carriers of FV Leiden (20 men and 20 women). Specificity and sensitivity of the assay were 98% and 95%, respectively. The normal range was calculated by measuring APC resistance in 118 healthy individuals. Results were log transformed, outliers were eliminated, and the mean ±2 standard deviations (SD) of the ratios was calculated, giving a range of 0.81 to 1.35. A modification of the functional assay, reported to have improved specificity in comparison to the classical method, was also used as a confirmatory test.13 It requires predilution (1:5) of the samples in plasma depleted of FV before testing in the functional APC resistance assay.
Mixing experiments.Mixtures of plasmas from patients and control subjects (1:1) were tested in the functional APC resistance assay and ratios were calculated. Plain plasmas from individuals homozygous for the FV 506Q or for the R2 polymorphism and a normal plasma were tested in parallel. The aim of these studies was to artificially create a sample doubly heterozygous for the R2 and the FV Leiden mutations and to compare its ratio with that of controls.
Time-course of FV degradation.To test if FV expressed by the gene encoding the R2 polymorphism had normal stability in plasma, the coagulant activity of FV as a function of time was measured in the plasma from a patient homozygous for the R2 polymorphism, from a patient heterozygous for FV R506Q, and in control plasma. Plasma samples were kept at 37°C throughout the experiment. Aliquots of 100 μL were drawn from each plasma at 30, 60, 90, 120, 150, 180, 210, 240, and 300 minutes, and the FV activity against reference plasma was measured. The experiment was performed on three separate occasions. Results were expressed as mean percentage ±SD of baseline FV observed at time zero in each plasma.
Polymerase chain reaction (PCR) amplification and polymorphism analysis.Genomic DNA (100 ng) was amplified with Taq polymerase (1.3 U) in 30 cycles. Primers for the amplification of exon 13 (numbered in Fig 1) were constructed from the FV cDNA sequence14 and have been previously described.11,12 Fragments 1-2 (nt 2182-2889) and 3-4 (nt 3579-4280) were amplified in the same conditions: 20 seconds of denaturation at 93°C, 15 seconds of annealing at 52°C, and 70 seconds of extension at 70°C. In subjects heterozygous for more than one polymorphism of exon 13, the allelic linkage was determined by the use of a PCR fragment (1-4, nt 2182-4280) containing all of the markers under study. Primers 1 and 4 were used in the following conditions: 20 seconds of denaturation at 93°C, 30 seconds of annealing at 58°C, and 2 minutes of extension at 70°C. Genotyping at the Met/Val polymorphism in exon 1615 was performed by allele-specific amplification (ASA-PCR). Allelespecific oligonucleotides (5′CTACATAAGGACAGCAACATACCTA3′ nt 5356-5380 and 5′CATAAGGACAGCAACATACCTG3′ nt 5359-5380) based on the published cDNA sequence14 and bearing one mismatch five nucleotides upstream the 3′ terminus were designed for both alleles of this polymorphic site. The reverse primer in intron 16 (5′TGTCTCAGAAGCATCTCATGT3′) was derived from sequencing in our laboratory (unpublished results). Selective amplification, checked by sequencing, occurred in the following conditions: 0.05 mmol/L dNTPs, 2 mmol/L MgCl2 , 4% dimethyl sulfoxide (DMSO), and 15 seconds of annealing at 58°C. FV R506Q was determined as previously described.16
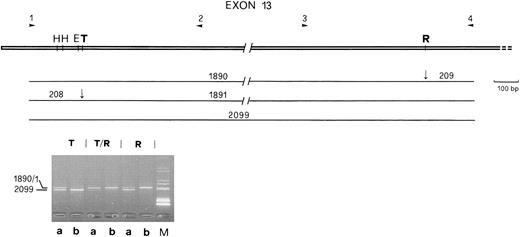
(Upper part) Localization of the polymorphic sites and of primers used to define haplotypes in exon 13 of the FV gene. H, HinfI; E, EcoRI; T, Taq I; R, Rsa I. (Lower part) Single (T and R) and combined (T/R) restriction of the large fragment (2,099 bp) for the assignment of linkage between polymorphisms in a doubly heterozygous subject (a, Indian no. 5); b, homozygous subject used as control (Indian no. 33 in Table 1). The restriction map for each allele is reported above the gel. The band corresponding to the 208/9-bp fragments is not shown. M, molecular weight marker. Fragment size is given in basepairs.
(Upper part) Localization of the polymorphic sites and of primers used to define haplotypes in exon 13 of the FV gene. H, HinfI; E, EcoRI; T, Taq I; R, Rsa I. (Lower part) Single (T and R) and combined (T/R) restriction of the large fragment (2,099 bp) for the assignment of linkage between polymorphisms in a doubly heterozygous subject (a, Indian no. 5); b, homozygous subject used as control (Indian no. 33 in Table 1). The restriction map for each allele is reported above the gel. The band corresponding to the 208/9-bp fragments is not shown. M, molecular weight marker. Fragment size is given in basepairs.
In doubly heterozygous subjects, the allelic linkage between exon 13 and exon 16 polymorphisms was estimated by subtraction of the invariant FV 506Q haplotype, present in Italian carriers of the R506Q mutation8,17 or by likelihood methods.18
Restriction analysis.PCR products were digested under the conditions recommended by the suppliers (Boehringer Mannheim [Mannheim, Germany] and Promega [Madison, WI]).
Sequencing.The amplified fragment containing the exon 16 polymorphism was excised from low melting point agarose gel and sequenced as control with Sequenase (US Biochemical, Cleveland, OH). 35S-α dATP was used as the radio-label.
PATIENTS AND CONTROLS
The Hemophilia and Thrombosis Center in Milan is a tertiary referral center that receives patients mainly from Northern Italy, although patients from Central and Southern Italy are not uncommon. Patients are generally referred by their family physicians or from district hospitals. In all patients referred for a thrombotic episode, a complete thrombophilia screening is performed.19
Patients heterozygous for the R506Q mutation.Patients with resistance to APC and heterozygous for the FV R506Q mutation who were diagnosed between 1993 and 1996 were considered. The distribution of normalized ratios obtained with the functional test for APC resistance was analyzed and 22 patients with ratios below the 15th percentile (ratio, < 0.57), 14 patients with ratios between the 15th and the 25th percentile (0.57 ≤ ratio < 0.61), and 31 patients with ratios above the 70th percentile (ratio, ≥ 0.67) were selected for DNA studies.
Patients functionally resistant to APC.The test used in our laboratory to screen for APC resistance is highly sensitive but has limited specificity when evaluated against the presence of the R506Q mutation, which leads to diagnosing APC resistance in patients without this genetic defect. Although a proportion of these patients turn out to have transient alterations that influence the functional assay (elevated factor VIII levels or pregnancy), others have persistently low ratios at repeated testing with no apparent cause. Moreover, when these patients are tested with the more specific functional assay that includes a predilution of the test sample in FV-deficient plasma,13 the ratios obtained are still below the normal range, whereas this is not observed for transient alterations such as those mentioned above. These patients with persistent APC resistance in the absence of the R506Q mutation are provisionally termed functionally APC resistant throughout this report. They represent approximately 5% of patients with venous thromboembolism referred for thrombophilia screening and with an abnormal result of the test for APC resistance. Ten of these patients functionally resistant to APC were investigated in this study.
Patients and normal controls selected from a population-based case control study.The first 100 consecutive patients with an objectively confirmed episode of venous thrombosis referred to the Thrombosis Center in 1996 were recruited for the study. Controls were healthy individuals asked to come to the Thrombosis Center to give a blood sample, were matched by sex and age to the cases, had a negative personal history of thrombosis, and had no renal, liver, or neoplastic disease at the time of blood sampling.
Other populations.DNA studies were also performed in 120 healthy Italian individuals, 40 Somali, and 40 Indians. Among them, FV haplotype studies were performed on 12 subjects of Italian origin, 7 Somali, and 7 Indians.
RESULTS
Definition of a FV polymorphic haplotype in control populations.FV gene haplotypes were constructed using five restriction polymorphisms11,12 in exon 13 (Table 1) and a sequence variation located in exon 1615 (Table 1). The exon 13 markers included the Rsa I polymorphic site, the rare allele of which (R2) has been previously found to be associated with partial FV deficiency in the Italian population.12
The detection of all exon 13 polymorphisms in a large PCR-amplified fragment (Fig 1) enabled us to determine unambiguously their allelic linkage. Allelic linkage between exon 13 and exon 16 polymorphisms, which are 13 kb apart in the FV gene, was clearly established in homozygotes. In doubly heterozygous patients or controls, linkage was estimated by subtraction of the invariant FV 506Q haplotype or by likelihood methods.18 A complete linkage disequilibrium between the EcoRI and Rsa I polymorphic sites was observed. These haplotype studies defined in all subjects carrying the R2 allele the invariant presence of the HR2 haplotype (Table 1).
Among the sequence variations that make up the HR2 haplotype, two cause aminoacid substitutions, His1299Arg (R2) and Met1736Val,15 and four are synonymous. Direct sequencing of parts of exon 13 in two HR2 homozygous subjects indicated that this haplotype also included the aminoacid substitutions Arg830Lys, Arg837His, and Leu1257Ile, corresponding to polymorphic sites already described.11 12 Sequencing of exons (7, 10, and 16 through 19) coding for FV regions known to interact with APC20 excluded the presence of additional gene variations.
FV genotype studies in selected APC resistant patients.DNA studies were performed in 67 patients carrying the FV R506Q mutation: 22 with APC ratios below the 15th percentile, 14 with APC ratios between the 15th and the 25th percentiles, and 31 with APC ratios above the 70th percentile. The R2 allele was found in the heterozygous condition in 8 patients, 6 belonging to the group (Fig 2) with the lowest ratios (<0.57), and 2 (not shown) with APC ratios of 0.57 (16th percentile) and 0.59 (21st percentile), respectively. The frequency of the R2 allele was significantly different between patients selected in the lowest or highest percentiles (P < .01).
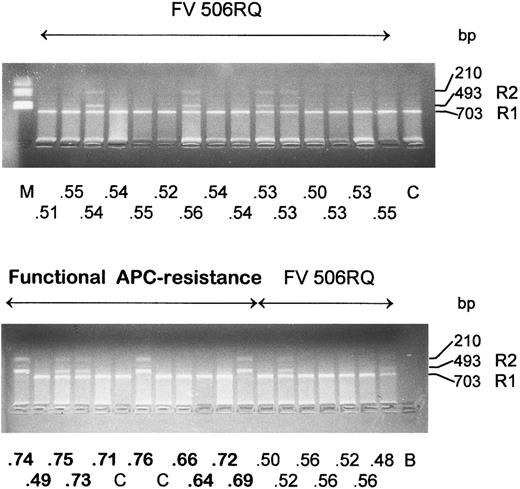
Rsa I restriction analysis in two groups of Italian patients, 22 carriers of the Leiden mutation (FV 506RQ) with APC ratio < 0.57 (below the 15th percentile) and 10 functionally APC resistant patients. The APC ratio value is reported for each patient under the corresponding lane. Size of the restriction fragments that identify the R1 and R2 alleles are indicated. C, normal controls; B, amplification without template DNA; M, molecular weight marker.
Rsa I restriction analysis in two groups of Italian patients, 22 carriers of the Leiden mutation (FV 506RQ) with APC ratio < 0.57 (below the 15th percentile) and 10 functionally APC resistant patients. The APC ratio value is reported for each patient under the corresponding lane. Size of the restriction fragments that identify the R1 and R2 alleles are indicated. C, normal controls; B, amplification without template DNA; M, molecular weight marker.
Ten patients functionally resistant to APC in the absence of the FV R506Q mutation were also studied: 3 were homozygous for the R2 allele and 2 were heterozygous (Fig 2). The homozygous subjects were about 50 times more frequent (P < .001) than expected from the frequency of the R2 allele in the general population. The presence of the HR2 haplotype was confirmed in patients (patient BA in Table 1), as was the haplotype invariantly associated with the FV R506Q mutation.8
APC-ratios in cohorts of patients and controls selected for the HR2 haplotype.The presence of the R2 allele was investigated in 100 patients with venous thromboembolism and in 98 age- and sex-matched controls. A similar number of patients (11; 2 of them were on anticoagulants) and controls (9) carried this allele that was invariantly associated with the HR2 haplotype. Mean APC ratios determined in these HR2 carriers, 9 patients (0.86 ± 0.1 SD) and all 9 controls (0.93 ± 0.1 SD), were lower than those obtained in 30 patients (0.96 ± 0.23 SD) and 30 controls (1.01 ± 0.16 SD) randomly selected among noncarriers. Although differences within patients or controls were not statistically significant (t-test for equality of means: patients, P = .08; controls, P = .09) due to their low numbers, when all carriers were pooled and compared with noncarriers, a significant difference (P = .01) was observed. FV activity could be evaluated in 5 patients and 9 control subjects carrying the HR2 haplotype; all of them showed normal values, with mean FV activity of 110 (±10 SD) and 126 (±24 SD), respectively.
Plasma mixing experiments and time course of the FV activity.To confirm the results obtained in the functional APC resistance assay of doubly heterozygous patients and to further investigate the interaction of the FV bearing the R506Q mutation with FV produced by the gene marked by the HR2 haplotype, plasmas from 2 patients homozygous for the R506Q mutation and for the HR2 haplotype, respectively, were mixed in equal amounts. Results of the APC resistance assays are summarized in Table 2. The artificial double heterozygote showed an APC ratio lower than the unmixed heterozygote or than the mixture of normal plasma with that from the patient homozygous for the R506Q mutation.
The stability in plasma of FV from an HR2 homozygous patient was compared with that of FV from a R506Q heterozygote and from a normal control (Table 3). No significant differences were observed in a time-course experiment evaluating the degradation of FV at 37°C.
Dating of the HR2 haplotype.To estimate the age and geographical distribution of the HR2 haplotype and to make a comparison with the FV 506Q,21 the presence of the R2 allele was investigated in two other populations with very different genetic backgrounds.22 Screening of 40 control subjects from Somalia and 40 from Southern India showed that the frequency of the R2 marker in Somali (7/80 [0.08]) and Indians (8/78 [0.10]) was similar to that observed in Italians (20/240 [0.08]). Moreover, the HR2 haplotype was invariantly found to underlie the R2 marker in these populations (Table 1).
DISCUSSION
Up to now, the FV R506Q mutation in the FV gene is the only genetic defect for which a causal relationship to APC resistance has been clearly established. The occurrence of APC resistance in the absence of this mutation and the variability of the APC resistance phenotype in heterozygotes for the R506Q mutation suggest the possibility that other gene variations may be responsible for or contribute to APC resistance. Haplotype analysis, a powerful tool to investigate gene variation and to establish its association with phenotypes, defined a new FV genetic component, marked by the HR2 haplotype and different from the R506Q mutation, that contributes to the APC resistance phenotype in patients and normal controls.
The relationship between the HR2 haplotype and the APC resistance phenotype was clearly established by finding 3 HR2 homozygotes and 2 heterozygotes among 10 patients functionally resistant to APC, selected after repeated testing meant to rule out known acquired causes of APC resistance and confirmed with a more specific functional assay. These figures are significantly higher than those predicted on the basis of the R2 allele frequency in the general population.
In the Italian population, the gene frequencies of the HR2 haplotype and of the R506Q mutation that do not reside on the same chromosome predict that about 1/1,000 individuals is doubly heterozygous. The distribution of the HR2 haplotype and its relationship with the APC resistance phenotype were evaluated in groups of patients with venous thromboembolism selected for their different degrees of APC resistance. In a group of 22 patients heterozygous for the R506Q mutation and selected for particularly low APC ratios, we found 6 double heterozygotes. A parallel study conducted on 10 patients with low ratios and 10 with high ratios from a different center (G. Castaman, unpublished results) detected 2 additional doubly heterozygous patients, both of them in the low ratio group. The in vitro mixing studies showed that an artificially created double heterozygote has a ratio lower than a simple R506Q heterozygote, thus confirming the results obtained in the functional APC resistance assay in doubly heterozygous patients. The mixing of plasmas from homozygous patients is a sort of functional complementation test, the results of which are compatible with alleles at the same locus giving rise to the observed phenotype.
The contribution of the HR2 haplotype to the APC resistance phenotype was also detectable in patients with venous thromboembolism and controls from a case control study who, however, showed similar frequencies of the HR2 haplotype.
Taken together, these findings suggest that the FV gene marked by the HR2 haplotype is both able to contribute by itself to determine a mild APC resistance phenotype and to interact synergically with the R506Q mutation to produce a severe APC resistance phenotype. The doubly heterozygous condition would produce effects marked enough to be usually shown, whereas the heterozygous or homozygous condition for HR2 would go frequently undetected by the APC resistance assay due to its minimal expression at the phenotypic level. The population-based case control study indicates that heterozygosity for HR2 is not by itself a risk factor for venous thrombosis; however, a much larger sample is needed to estimate the potential risk associated with the rare (2 to 5/1,000) homozygous condition.
The mechanism through which the FV gene marked by the HR2 haplotype may produce its phenotypic effects is likely based on the several aminoacid substitutions predicted by this haplotype. Most changes are located in the heavily glycosylated B domain,23 whose fragments, derived from the APC-mediated cleavage of intact FV, have been directly implicated in the protein C anticoagulant pathway.24 A pseudo-homozygous APC resistance mechanism9 10 could be excluded in our study for three reasons: the mild APC resistance found in the HR2 homozygotes, the normal levels of FV activity in their plasma, and the FV stability in time-course experiments.
The normal FV level found in patients and controls heterozygous or homozygous for the R2 allele does not conflict with our previous finding12 of this allele associated with low FV levels in a cohort of Italian subjects selected for partial FV deficiency. In the same study,12 a subject with the R2 polymorphism and high FV level was present, which suggested that the R2 allele, although in incomplete linkage with an FV defect, was not causative of it. Hence, the present findings may simply reflect the different recruitment criteria, namely low APC ratio or the presence of the HR2 haplotype.
Tracing of the 506Q allele and its genetic background in various populations has provided important insights into the origin of this pathologically relevant mutation.21 In contrast to the FV 506Q gene, which is confined to Caucasoid populations,25 the invariant HR2 haplotype was found with similar frequencies in the Italian population and in subjects of Somali and Indian origin, suggesting a worldwide distribution. Because modern Somali are representative of the original population that spawned the migration from Africa more than 100,000 years ago,22 our results indicate ancestral mutational events as the origin of the HR2 haplotype, dating it further back than the R506Q mutation.
Supported by Telethon-Italy (Grant No. E125).
Address reprint requests to F. Bernardi, BS, Dipartimento di Biochimica e Biologia Molecolare, Università degli Studi di Ferrara, Via L. Borsari 46, 44100 Ferrara, Italy.