Abstract
We recently found that retinoic acids (RAs) exert anticoagulant effects by upregulating thrombomodulin (TM) and downregulating tissue factor (TF ) expression in acute promyelocytic leukemia (APL) cells and monoblastic leukemia cells. Two classes of nuclear RA receptors, termed retinoic acid receptors (RARs) and retinoid X receptors, have been identified. Each receptor class consists of three subtypes. In the present study, we have used several synthetic retinoids to determine which receptor subtypes are involved in the regulation of TM and TF expression in NB4 APL cells, U937 monoblastic leukemia cells, and human umbilical vein endothelial cells (HUVECs). Am80, which has no binding affinity for RARγ, and Ch55, which does not bind to cytoplasmic retinoic acid binding protein (CRABP), upregulated TM and downregulated TF in NB4 and U937 cells, similar to all-trans RA (ATRA). A specific RARα antagonist, Ro41-5253, significantly suppressed the upregulation of TM by ATRA and Am80 in NB4 cells, U937 cells, and HUVECs. In contrast, only with preincubation with both RARα and RARβ antagonists was downregulation of TF by retinoids suppressed in NB4 cells. These findings indicate that the mechanism of transactivation and transrepression functions of RARs are distinct and also elucidate the major role of RARα in TM upregulation by retinoids in leukemic cells and HUVECs and the cooperation of RARα and RARβ in TF downregulation by retinoids. They also indicate that binding to CRABP is not required for the anticoagulant effect of retinoids and that synthetic retinoids will prove very useful in controlling distinct targets, the TM and TF genes, at the level of transcription, and will permit the development of retinoids with a new type of anticoagulant effect.
VITAMIN A (RETINOL) and its biologically active derivatives (collectively referred to as retinoids), the physiologically most important of which are retinoic acids (RAs), exert pleiotropic effects on vertebrate development, cellular differentiation, and homeostasis.
The biologic effects of RAs are mediated by their binding to receptors. Two classes of nuclear RA receptors, termed retinoic acid receptors (RARs) and retinoid X receptors (RXRs), have been identified and cloned.1 Each class of receptor includes three receptor subtypes, termed α, β, and γ, and their isoforms. RARα is the most ubiquitously expressed in human tissues, whereas RARβ and RARγ display more restricted patterns of distribution. RARγ is predominantly expressed in skin.2 RARs and RXRs differ substantially in primary structure and in their response to synthetic retinoids. The discovery of synthetic retinoids that have specific binding affinities for the different subtypes of RARs and RXRs would increase the understanding of the roles of the receptors in governing the biologic effects of retinoids on leukemic cells3 and vascular endothelial cells. Several synthetic retinoids with agonistic and antagonistic effects on the activation of the respective receptors have been created.
We recently found that RAs exert anticoagulant effects by upregulating thrombomodulin (TM) and downregulating tissue factor (TF ) expression in acute promyelocytic leukemia (APL) and monoblastic leukemia cells.4 5 In the present study, we used several synthetic retinoids to determine which receptor subtypes are involved in the regulation of TM and TF expression in APL cells, monoblastic leukemia cells, and human umbilical vein endothelial cells (HUVECs).
MATERIALS AND METHODS
Reagents.Chemicals were purchased from the following suppliers. ATRA, porcine mucosal heparin, and human-thrombin (3,120 NIHU/mg protein) were from Sigma (St Louis, MO). Ro41-5253 and Ro25-7386 were kindly provided by F. Hoffman-La Roche, Ltd (Basel, Switzerland). Am80, Ch55, and LE540 were synthethized as previously reported.6 ATRA binds to all RAR subtypes but does not bind to RXRs.1 Am80 does not bind to RARγ and only weakly binds to cytoplasmic retinoic acid binding protein (CRABP). Ch55 has higher affinity for RARα, β, and γ than does ATRA, and does not bind to CRABP. LE540 is a specific RARβ antagonist, being a benzo-homologue of LE135.7 Ro41-5253 is a selective RARα antagonist.8,9 Ro25-7386 is a specific RXR agonist and does not bind to RARs at all. Comparisons of the binding affinities of these retinoids for RARs, RXRs, and CRABP are briefly summarized in Table 1. Recombinant human soluble TM, which possesses the entire extracellular TM domain, was a gift from Asahi Chemical Industry Co (Fuji, Japan). Human placenta-derived TF (Thromborel S) was from Behringwerke AG (Marburg, Germany). The chromogenic substrate S2266 was from Chromogenix (Stockholm, Sweden). Human protein C (inactivated and activated) and antithrombin were generously provided by the Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan) and Green Cross (Osaka, Japan), respectively. Monoclonal mouse antihuman TM IgG KA-4 and polyclonal rabbit antihuman TM antibody were from Teijin, Ltd (Tokyo, Japan). KA-4 recognizes the serine/threonine-rich polypeptide core near the primary thrombin binding site of the TM molecule.10 Monoclonal mouse antihuman TF IgG 5G9 was from Corvas International, Inc (La Jolla, CA). 5G9 rapidly inactivates the TF/factor VIIa (FVIIa) complex before dissociation of FVIIa by blocking access of macromolecular substrates (FVIIa, IX, and X) to the TF/FVIIa complex.11 All other chemicals were reagent-grade products and purchased from Wako Pure Chemicals (Osaka, Japan), unless otherwise indicated.
Cell culture.The following human leukemic cell lines were used in this study. An APL cell line, NB4, was kindly provided by Dr M. Lanotte (Hôpital Saint Louis, Paris, France). NB4 is characterized by the presence of chromosomal translocation t(15; 17) with rearrangements of the RARα and promyelocytic leukemia (PML) genes, as observed in most cases with APL.12 13 A monoblastic leukemia cell line, U937, was provided by the Japanese Cancer Research Bank (Tokyo, Japan). Each cell line and HUVECs were cultured as already described. All retinoids were dissolved in absolute ethanol and further diluted into growth media to the desired concentration so that the final ethanol concentration in the culture media was less than 0.1%, at which cells exhibited no damage. No retinoids used in this study showed an effect on cell viability within 24 hours. The control leukemia cells and HUVECs incubated without retinoids were exposed to an appropriate amount of ethanol in the culture media. All the procedures involving retinoids were performed under subdued light.
Measurement of TM and TF antigens.Leukemic cells were incubated with the retinoid reagents for 24 hours and then washed with phosphate-buffered saline (PBS) three times. Cell numbers were counted and adjusted. Cells were then extracted for 30 minutes with 0.5% Triton X-100 in PBS at 4°C. Cellular debris was removed by centrifugation at 12,000g for 20 minutes. Cell lysates were stored at −80°C until assay. Total TM and TF antigens in cell lysates were measured by enzyme-linked immunosorbent assay, as already reported.4 14
Cell surface TM cofactor activity.Cell surface TM cofactor activity was measured as previously described.4 10 This assay measures the ability of the intact cells, in the presence of thrombin (3.3 NIHU/mL) and Ca2+ (1.3 mmol/L), to activate exogenous protein C (0.16 μmol/L) and thus develop activated protein C activity to cleave the small molecular weight substrate S-2266. The results were expressed as ΔOD405nm per minute or as the percentage of the initial velocity of activated protein C formation (with 100% considered the rate of formation with cell surface TM under basal conditions). Controls with cells in the absence of thrombin and protein C were treated similarly, and no activation of protein C was observed.
Cell surface TF cofactor activity: analysis of procoagulant activity in clotting assays.Leukemic cell suspensions were adjusted to 1 × 107/mL in PBS. Cells (1 × 106) were added to 0.1 mL of pooled human normal plasma. After incubation at 37°C for 3 minutes, 0.1 mL of 25 mmol/L calcium chloride was added and plasma recalcification time was determined with a CA-100 semiautomatic coagulometer (Sysmex, Kobe, Japan). Our previous study showed that the procoagulant activities on the surface of NB4 and U937 cells determined as described above are deduced from TF expression,4 and thus prolongation of recalcification time is mainly due to downregulation of TF expression by retinoids. TF cofactor activity was quantitated by reference to standard curves (log-log plot) constructed with human placenta TF and TF activity that yielded a 50-second recalcification time and was defined as 1 U/mL.
Immunoblotting analysis.Western blot analysis of TM and TF in cell lysates was performed essentially as previously described.5 Horseradish peroxidase-conjugated KA-4 anti-TM monoclonal antibody and 5G9 anti-TF monoclonal antibody were used for the detection of TM and TF, respectively. The exact same amount of lysates from 5 × 106 cells was applied to each lane.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR).Measurement of TM and TF mRNA levels in leukemia cells was performed by quantitative RT-PCR assays using the SuperScript Preamplification system (Life Technologies, Inc, Gaithersburg, MD).15 16 The sequences for sense and antisense primers for TM were 5′ CAT GTG CGA GAC CGG CTA CCG GCT GGC GG 3′, corresponding to nucleotides 1072-1100, and 5′ AGG GGC TGG CAC TGG TAC TCG CAG TTG GC 3′, corresponding to nucleotides 1262-1290. The sequences for sense and antisense primers for TF were 5′ CTA CTG TTT CAG TGT TCA AGC AGT GA 3′, corresponding to nucleotides 723-748, and 5′ CAG TGC AAT ATA GCA TTT GCA GTA GC 3′, corresponding to nucleotides 980-1005. The repeated PCR cycles established were 28.
RESULTS
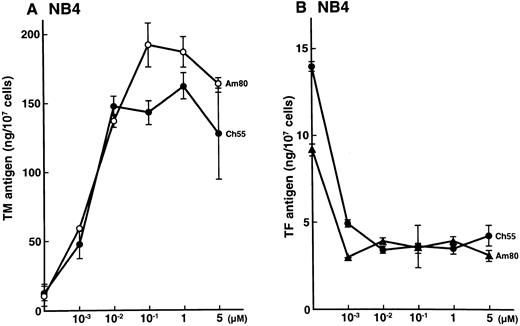
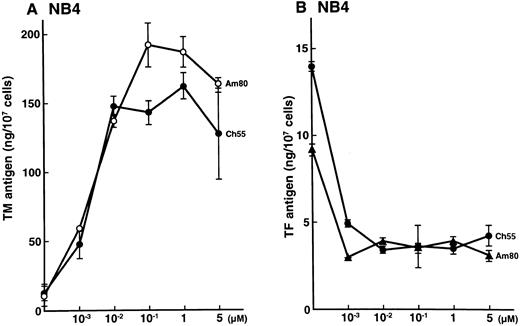
Effects of synthetic retinoids on total TM and TF antigens in NB4 cells.NB4 cells were incubated for 24 hours with various concentrations of Am80 and Ch55, as indicated in Fig 1. Am80 and Ch55 increased the TM antigen level (Fig 1A) while decreasing the TF antigen level (Fig 1B) in a dose-dependent manner (up to 0.1 μmol/L). These effects were similar to those previously reported for ATRA and 9-cis RA on NB4 cells.4 5 The specific RXR agonist Ro25-7386 slightly increased the TM antigen level (2-fold increase) only with incubation with an excess amount (1 μmol/L; data not shown).
Effects of synthetic retinoids on total TM and TF antigens in NB4 cells. Dose-dependent effects of Am80 and Ch55 on TM (A) and TF (B) antigen expression in NB4 cells. NB4 cells were incubated with Am80 or Ch55 (0.001 to 5 μmol/L) for 24 hours.
Effects of synthetic retinoids on total TM and TF antigens in NB4 cells. Dose-dependent effects of Am80 and Ch55 on TM (A) and TF (B) antigen expression in NB4 cells. NB4 cells were incubated with Am80 or Ch55 (0.001 to 5 μmol/L) for 24 hours.
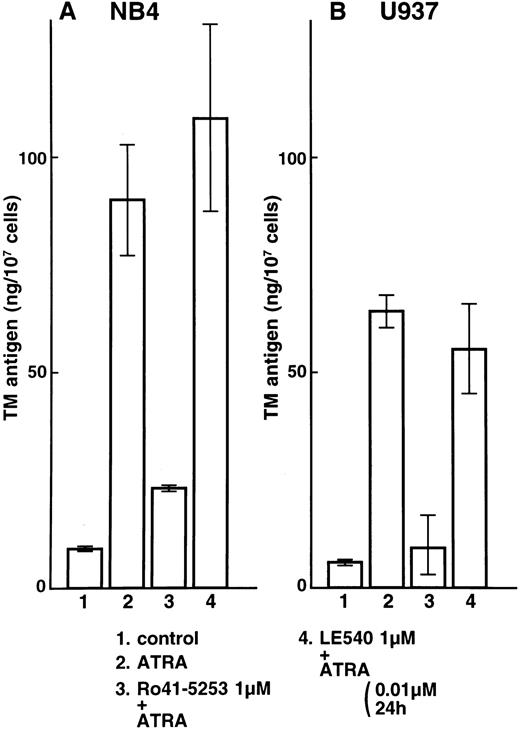
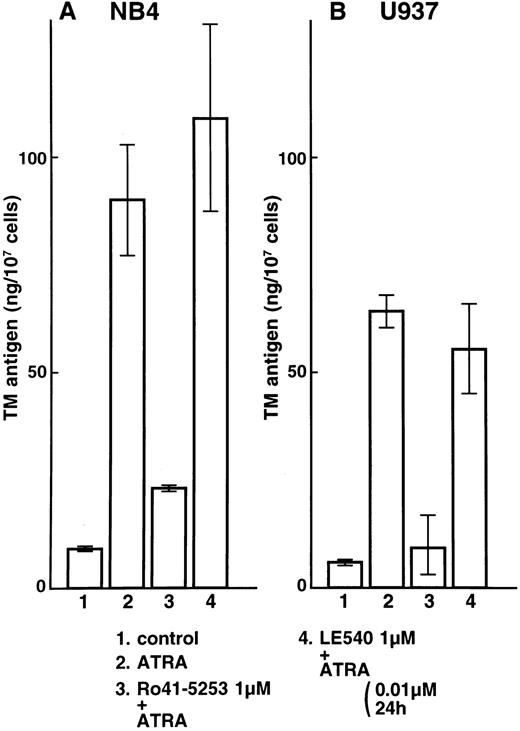
Effects of the specific RARα antagonist Ro41-5253 and the RARβ antagonist LE540 on total TM and TF antigens in NB4 and U937 cells.NB4 and U937 cells were then incubated with Ro41-5253 (1 μmol/L) only or with ATRA (0.01 μmol/L) added after preincubation with Ro41-5253 for 1 hour. The antagonist (1 μmol/L) alone, in the absence of retinoids, did not have appreciable effects on TM or TF expression in NB4 or U937 cells. Ro41-5253 caused a dose-dependent reduction of the TM upregulation and TF downregulation induced by ATRA (data not shown). A 10-fold excess concentration of Ro41-5253 to ATRA was required to counteract the effects of ATRA. A 100-fold excess of Ro41-5253 added for the 1-hour period preceding the addition of ATRA significantly suppressed upregulation of TM antigen by ATRA in NB4 and U937 cells (Fig 2A and B, lane 3). The RARβ antagonist LE540 had little effect on the TM upregulation by ATRA (Fig 2A and B, lane 4). These findings suggest that RARα is an important mediator of TM upregulation.
Selective counteractive effect of RARα antagonist on TM upregulation by ATRA in NB4 (A) and U937 (B) cells. NB4 and U937 cells were incubated with ATRA (0.01 μmol/L) for 24 hours (lane 2). These cells were also preincubated with 1 μmol/L Ro41-5253 (lane 3) or LE540 (lane 4) for 1 hour. Lane 1 shows control without treatment.
Selective counteractive effect of RARα antagonist on TM upregulation by ATRA in NB4 (A) and U937 (B) cells. NB4 and U937 cells were incubated with ATRA (0.01 μmol/L) for 24 hours (lane 2). These cells were also preincubated with 1 μmol/L Ro41-5253 (lane 3) or LE540 (lane 4) for 1 hour. Lane 1 shows control without treatment.
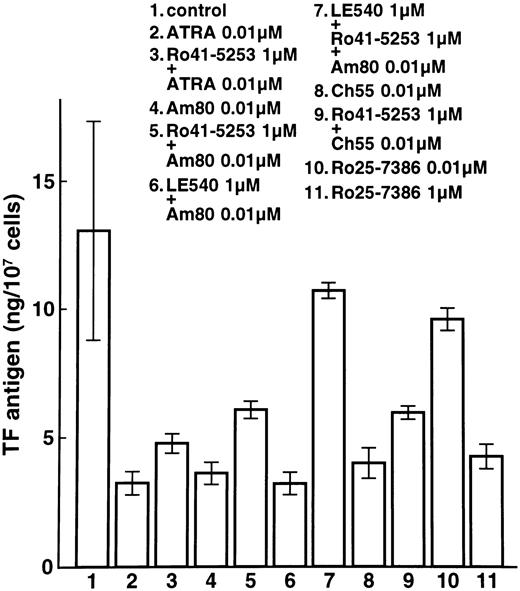
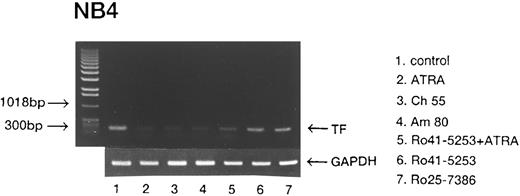
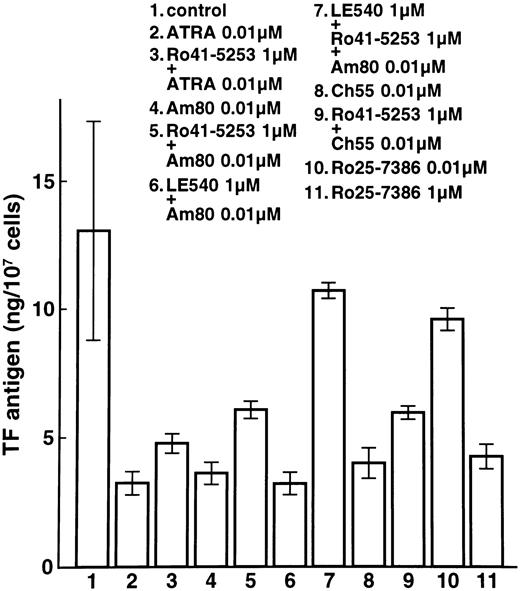
We next examined whether RARα also plays a crucial role in the downregulation of TF. Expression of TF was downregulated by incubation with ATRA, Am80, and Ch55 (Fig 3, lanes 2, 4, and 8). Despite preincubation with Ro41-5253 or LE540, TF antigen was downregulated significantly by ATRA and the synthetic retinoids Am80 and Ch55 in NB4 cells (Fig 3, lanes 3, 5, 6, and 9). Similar effects were observed on TF antigen levels in U937 cells (data not shown). With preincubation with both Ro41-5253 and LE540, downregulation of TF by Am80 was significantly counteracted (Fig 3, lane 7). These findings indicate that both RARα and RARβ are necessary for TF downregulation by ATRA, Am80, and Ch55. When incubation was performed with an excess amount (1 μmol/L) of Ro25-7386, reduction in TF was similar to that obtained with 0.01 μmol/L Ch55 (Fig 3, lanes 10 and 11). High concentrations of Ro25-7386 appear to downregulate TF expression independent of exogenous RAR ligands.
Effects of ATRA and synthetic retinoids on total TF antigen in NB4 cells. NB4 cells were incubated with 0.01 μmol/L ATRA (lane 2), Am80 (lane 4), Ch55 (lane 8), Ro25-7386 (lane 10), or 1 μmol/L Ro25-7386 (lane 11). Cells were also incubated with 1 μmol/L Ro41-5253 (lane 5), LE540 (lane 6), or both Ro41-5253 and LE540 (lane 7). One hour after the preincubation, 0.01 μmol/L ATRA (lane 3), Am80 (lanes 5, 6, and 7), or Ch55 (lane 9) was added for 24 hours. Lane 1 shows control without treatment.
Effects of ATRA and synthetic retinoids on total TF antigen in NB4 cells. NB4 cells were incubated with 0.01 μmol/L ATRA (lane 2), Am80 (lane 4), Ch55 (lane 8), Ro25-7386 (lane 10), or 1 μmol/L Ro25-7386 (lane 11). Cells were also incubated with 1 μmol/L Ro41-5253 (lane 5), LE540 (lane 6), or both Ro41-5253 and LE540 (lane 7). One hour after the preincubation, 0.01 μmol/L ATRA (lane 3), Am80 (lanes 5, 6, and 7), or Ch55 (lane 9) was added for 24 hours. Lane 1 shows control without treatment.
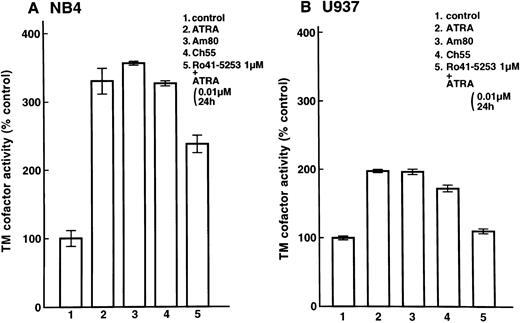
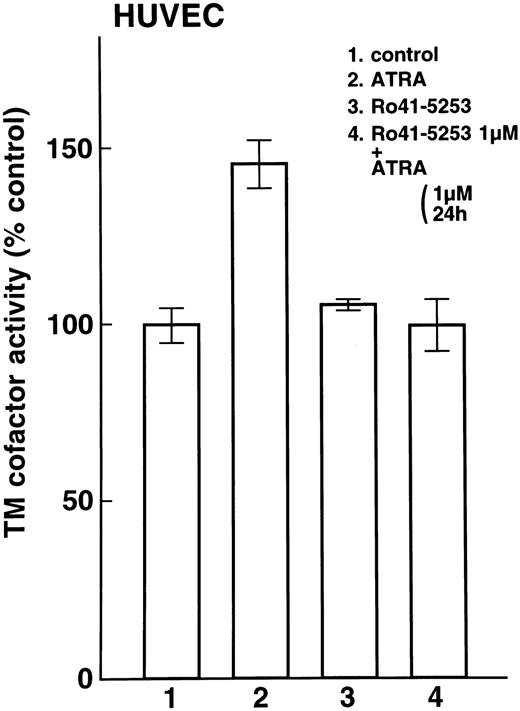
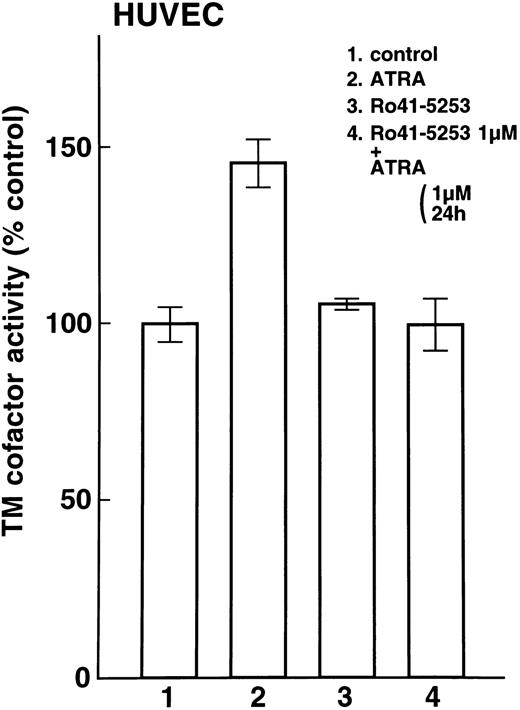
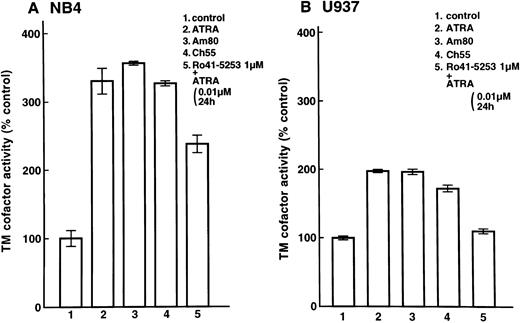
Enhancement of cell surface TM activity on NB4 and U937 cells and HUVECs by ATRA and synthetic retinoids.Cell surface TM activity was measured as the degree of acceleration of thrombin-catalyzed protein C activation. ATRA and synthetic retinoids increased the rate of protein C activation about 200% on NB4 cells (Fig 4A, lanes 2, 3, and 4) and about 100% on U937 cells (Fig 4B, lanes 2, 3, and 4). After pretreatment with Ro41-5253 for 1 hour, ATRA increased the rate of protein C activation about 100% on NB4 cell surfaces and only slightly on U937 cells (Fig 4A, lane 5, and Fig 4B, lane 5). When HUVECs were treated with ATRA, TM activity increased about 50% (Fig 5, lane 2). However, after preincubation with Ro41-5253 (1 μmol/L) for 1 hour, ATRA did not increase TM activity (Fig 5, lane 4).
Selective counteractive effect of RARα antagonist on upregulation of TM activity on leukemia cells. Changes in TM cofactor activity in protein C activation on NB4 (A) and U937 (B) cell surfaces were measured after exposure to 0.01 μmol/L ATRA (lane 2), Am80 (lane 3), or Ch55 (lane 4) for 24 hours. Cells were also incubated with Ro41-5253 (1 μmol/L), and ATRA (0.01 μmol/L) was added 1 hour later (lane 5). Basal ΔOD405nm per minute levels were 0.033 ± 0.002/min/5 × 106 NB4 cells (31 ± 2 ng activated protein C/106 cells) and 0.063 ± 0.002/min/5 × 106 U937 cells (61 ± 2 ng activated protein C/106 cells).
Selective counteractive effect of RARα antagonist on upregulation of TM activity on leukemia cells. Changes in TM cofactor activity in protein C activation on NB4 (A) and U937 (B) cell surfaces were measured after exposure to 0.01 μmol/L ATRA (lane 2), Am80 (lane 3), or Ch55 (lane 4) for 24 hours. Cells were also incubated with Ro41-5253 (1 μmol/L), and ATRA (0.01 μmol/L) was added 1 hour later (lane 5). Basal ΔOD405nm per minute levels were 0.033 ± 0.002/min/5 × 106 NB4 cells (31 ± 2 ng activated protein C/106 cells) and 0.063 ± 0.002/min/5 × 106 U937 cells (61 ± 2 ng activated protein C/106 cells).
Selective counteractive effect of RARα antagonist on upregulation of TM activity on HUVECs. Changes in TM cofactor activity in protein C activation on HUVECs were measured after exposure to 1 μmol/L ATRA (lane 2) or Ro41-5253 (lane 3) for 24 hours. Cells were also incubated with Ro41-5253 (1 μmol/L), and ATRA (1 μmol/L) was added 1 hour later (lane 4).
Selective counteractive effect of RARα antagonist on upregulation of TM activity on HUVECs. Changes in TM cofactor activity in protein C activation on HUVECs were measured after exposure to 1 μmol/L ATRA (lane 2) or Ro41-5253 (lane 3) for 24 hours. Cells were also incubated with Ro41-5253 (1 μmol/L), and ATRA (1 μmol/L) was added 1 hour later (lane 4).
Cell surface TF activity as measured by procoagulant activity.Treatment of NB4 cells with ATRA, Am80, and Ch55 decreased cell surface TF activity (Fig 6, lanes 2, 3, and 4). Ch55 was slightly more effective in decreasing TF activity than were ATRA and Am80. With pretreatment of Ro41-5253 for 1 hour, ATRA significantly reduced TF activity (Fig 6, lane 5). Ro41-5253 alone did not show any effect on cell surface TF activity.
Effect of RARα antagonist on downregulation of TF activity on NB4 cells. NB4 cells were incubated with ATRA (lane 2), Am80 (lane 3), or Ch55 (lane 4; 0.01 μmol/L). ATRA (0.01 μmol/L) was added 1 hour later to media containing Ro41-5253 (1 μmol/L; lane 5). Procoagulant activities on NB4 cells were measured as the recalcification time, as described in the Materials and Methods.
Effect of RARα antagonist on downregulation of TF activity on NB4 cells. NB4 cells were incubated with ATRA (lane 2), Am80 (lane 3), or Ch55 (lane 4; 0.01 μmol/L). ATRA (0.01 μmol/L) was added 1 hour later to media containing Ro41-5253 (1 μmol/L; lane 5). Procoagulant activities on NB4 cells were measured as the recalcification time, as described in the Materials and Methods.
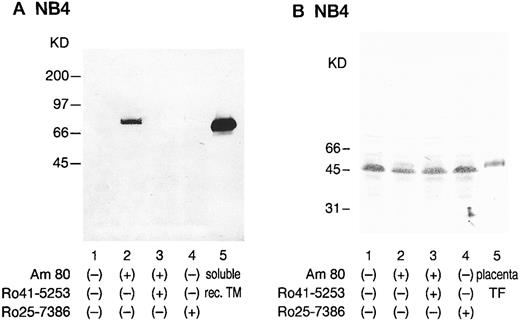
Western blot analysis with anti-TM and anti-TF antibodies.After treatment with Am80, the monoclonal antibody KA-4 identified the nonreduced form of TM as a prominent band at approximately 75 kD in NB4 cells (Fig 7A). This size was similar to that of placental and HUVEC-derived TM. Recombinant soluble TM exhibited a band at 65 kD. Ro41-5253 suppressed this marked increase in the TM band in NB4 cells. In contrast, after treatment with Am80, marked reduction in strength of the TF band at 45 kD in NB4 cells was observed (Fig 7B, lane 2). The NB4 cell TF molecule appears to be slightly smaller than placenta TF, probably due to differences in N-glycosylation, as recently suggested.17 Ro41-5253 only slightly counteracted the TF reduction by Am80 (Fig 7B, lane 3). The RXR specific agonist Ro25-7386 (0.01 μmol/L) did not exhibit an appreciable effect on TF expression (Fig 7B, lane 4).
Western blot analysis of NB4 cells modulated by synthetic retinoids with anti-TM and anti-TF antibodies. NB4 cell lysates (5 × 106 cells) were subjected to immunoblotting analysis using a monoclonal anti-TM antibody, KA-4 (A), and a monoclonal anti-TF antibody, 5G9 (B). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed under nonreducing conditions. NB4 cells were incubated with 0.01 μmol/L Am80 (lane 2) or Ro25-7386 (lane 4) for 24 hours. Selective counteractive effect of Ro41-5253 (1 μmol/L) on the effect of Am80 was also observed (lane 3). Lane 1 shows untreated NB4 cell lysate. Molecular weight markers are given along the left margin. Soluble recombinant TM (A; lane 5) and placenta TF (B; lane 5) were used as controls.
Western blot analysis of NB4 cells modulated by synthetic retinoids with anti-TM and anti-TF antibodies. NB4 cell lysates (5 × 106 cells) were subjected to immunoblotting analysis using a monoclonal anti-TM antibody, KA-4 (A), and a monoclonal anti-TF antibody, 5G9 (B). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed under nonreducing conditions. NB4 cells were incubated with 0.01 μmol/L Am80 (lane 2) or Ro25-7386 (lane 4) for 24 hours. Selective counteractive effect of Ro41-5253 (1 μmol/L) on the effect of Am80 was also observed (lane 3). Lane 1 shows untreated NB4 cell lysate. Molecular weight markers are given along the left margin. Soluble recombinant TM (A; lane 5) and placenta TF (B; lane 5) were used as controls.
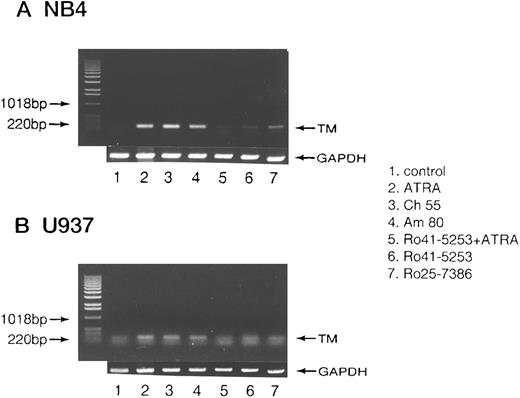
RT-PCR analysis of TM mRNA in leukemia cell lines.Increased expression of specific mRNA for TM was detected when NB4 (Fig 8A) or U937 cells (Fig 8B) were treated with 0.01 μmol/L ATRA or Am80 and Ch55 (Fig 8A and B, lanes 2, 3, and 4). However, no increase in TM mRNA level was detected after preincubation with Ro41-5253 for 1 hour (Fig 8A and B, lane 5). Ro25-7386 (0.01 μmol/L) slightly increased TM mRNA expression.
RT-PCR analysis of TM mRNA in NB4 cells modulated by synthetic retinoids. Total RNA was extracted from cultured NB4 (A) or U937 cells (B) after exposure to 0.01 μmol/L of ATRA (lane 2), Ch55 (lane 3), Am80 (lane 4), or Ro25-7386 (lane 7) and 1 μmol/L of Ro41-5253 (lane 6) for 5 hours. RT-PCR analysis of mRNA was performed as described in the Materials and Methods. Selective counteractive effect of Ro41-5253 (1 μmol/L) on the effect of ATRA was also observed (lane 5). Lane 1 represents untreated control. Basepair markers are given on the left.
RT-PCR analysis of TM mRNA in NB4 cells modulated by synthetic retinoids. Total RNA was extracted from cultured NB4 (A) or U937 cells (B) after exposure to 0.01 μmol/L of ATRA (lane 2), Ch55 (lane 3), Am80 (lane 4), or Ro25-7386 (lane 7) and 1 μmol/L of Ro41-5253 (lane 6) for 5 hours. RT-PCR analysis of mRNA was performed as described in the Materials and Methods. Selective counteractive effect of Ro41-5253 (1 μmol/L) on the effect of ATRA was also observed (lane 5). Lane 1 represents untreated control. Basepair markers are given on the left.
RT-PCR analysis of reduction of TF mRNA by synthetic retinoids.ATRA, Am80, and Ch55 (0.01 μmol/L) each markedly reduced the expression of TF mRNA in NB4 cells (Fig 9, lanes 2, 3, and 4). Ro41-5253 slightly counteracted the reduction of TF mRNA induced by ATRA (Fig 9, lane 5). Ro41-5253 (1 μmol/L) and Ro25-7386 (0.01 μmol/L) had no significant effects on TF mRNA expression.
RT-PCR analysis of TF mRNA in NB4 cells modulated by synthetic retinoids. NB4 cells were treated with various retinoids as depicted in Fig 8. RT-PCR analysis of TF mRNA was performed as described in the Materials and Methods.
RT-PCR analysis of TF mRNA in NB4 cells modulated by synthetic retinoids. NB4 cells were treated with various retinoids as depicted in Fig 8. RT-PCR analysis of TF mRNA was performed as described in the Materials and Methods.
DISCUSSION
In this study, we showed that TM upregulation by retinoids in leukemic cells and HUVECs was mediated by RARα and that TF downregulation was transduced by RARα, RARβ, and RXR. The activities of retinoids are mediated by the specific nuclear receptors RARs and RXRs. Retinoid activities are mediated principally through RAR/RXR heterodimers, and not RXR/RXR homodimers,3 because RAR suppresses ligand binding to RXR and its transactivation through allosteric interaction.18 When RAR is occupied by a ligand, RXR ligands activate the heterodimer.18 This may explain why the specific RXR agonist Ro25-7386 had little effect on TM transactivation. Retinoids induce transcriptional activation of TM expression through a specific DNA site, the RA-responsive element (RARE), in the 5′-flanking region of the TM gene,19 whereas they transrepress TF expression through binding to the transcriptional activation factor AP-1 (Jun/Fos) to retinoid-activated RARs.20 Because these two types of reactions differ in mechanism, it has been possible to dissociate the transactivation and transrepression functions of RARs using properly designed ligands.21
Similar potencies of three types of retinoids, ATRA, Am80, and Ch55, and their binding affinity for RARα suggest that RARα plays an important role in the process of TM induction in NB4 cells, U937 cells, and HUVECs if it is assumed that Am80 has no affinity for RARγ. Furthermore, the RARα antagonist Ro41-5253 counteracted the retinoid-induced transactivation of TM expression in these cells, but the RARβ antagonist LE540 did not, indicating that RARα plays a pivotal role in the TM transactivation system in these cells. This finding might have been expected, because suppression of RARβ gene expression associated with overexpression of RARα mRNA expression appears to be a general feature of hematopoietic cell lines22,23 and HUVECs.9 Under basal, ie, essentially retinoid-free, conditions, very low levels of RARβ mRNA are detected in HUVECs, whereas RARβ mRNA levels are increased threefold to fivefold in the presence of 1 μmol/L ATRA or Ch55.9 However, the RARα/RXR heterodimer may have higher affinity for the RARE of the TM gene than does the RARβ/RXR heterodimer. RARα expression alone does not appear to be a limiting factor for TM transactivation, because leukemia cells, such as K562 (erythroblastic) and TMD2 (B lymphoblastic) cells in which ATRA does not transactivate TM transcription,4 exhibit expression of RARα mRNA and protein.23 A selective cascade of interactions between receptor dimers, cofactors, silencing mediators for retinoid receptors,24 and members of the transcriptional machinery, such as TATA-binding proteins and TATA-associated factors, is necessary and these interactions may result from specific conformational change within the RAR/RXR.25 Further studies are necessary to elucidate the biologic complexity of TM transactivation.
In our study, an RARα antagonist only partially counteracted the TF downregulation induced by the specific RAR agonists ATRA, Am80 and Ch55 (Fig 3). This finding might also have been expected, because the transrepression functions of RARs for TF expression are distinct from the transactivation function of RARs in TM expression, as noted above. It has been reported that certain synthetic retinoids that are potent agonists for RARβ and RARγ but not for RARα inhibit AP-1–dependent gene expression through RARα.21 In this study, we have shown that the reduction of TF by Am80 was eliminated by preincubation with the combination of both RARα and RARβ antagonists. Furthermore, RXR agonist, which did not complex directly with RARs, had a mild downregulatory effect on TF expression. Upregulated RARα and β expression may be sufficient for AP-1 repression and RXR may facilitate negative repression, as suggested by a study of collagenase.26 It may also be possible that a high concentration of selective RXR agonist induces AP-1 repression without exogenous RAR agonist through its binding to RXR-RAR heterodimers.
Our findings also indicate that binding to CRABP by retinoids is not required for modulation of TM and TF synthesis, because Ch55 and Am80, which do not or only weakly bind to CRABP, are both effective modulators of TM and TF synthesis. The development of resistance to ATRA is an important limitation in differentiation therapy of APL. One of the mechanisms of acquired retinoid resistance is increased expression of CRABP, which may facilitate sequestration of retinoids to catabolic enzymes in the endoplasmic reticulum.27 Am80 is chemically more stable to light, heat, and oxidation than is ATRA. Furthermore, because Am80 has low affinity for CRABP and the anticoagulant effect of Am80 is similar to that of ATRA, as shown in this study, Am80 appears to be a promising new agent for patients with retinoid-resistant APL with increased CRABP levels due to its potential for overcoming the drug resistance created by RARγ,28 which is the major RAR in the dermal epithelium.3
ATRA is an effective downregulator of monocyte TF29 and of TF and cancer procoagulant in APL cells.4,5,30 Clinical application of retinoid is expected to be useful as a new strategy of anticoagulant therapy that changes the procoagulant character of cells including malignant cells, diseased endothelial cells,31 and monocytes by regulating TM and TF expression at the level of transcription. The growing knowledge of retinoid receptors and the signaling pathways of retinoid activity will ultimately permit rational design of tailor-made synthetic retinoids for medical indications including anticoagulant therapy.
ACKNOWLEDGMENT
The authors are indebted to Dr Shuji Tohda (Tokyo Medical and Dental University, Tokyo, Japan) for helpful discussions. The excellent skillful technical assistance of Kaori Okada is also gratefully acknowledged.
Supported in part by a research grant from the Ministry of Education, Science, Sports and Culture and a grant for intractable diseases from the Ministry of Health and Welfare of Japan.
Address reprint requests to Takatoshi Koyama, MD, The First Department of Internal Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima Bunkyo-ku, Tokyo, 113 Japan.