Abstract
It is essential for the study of T-cell function and the improvement of adoptive cell therapies to efficiently generate large populations of human primary T cells that reliably express foreign genes. This goal is achieved by using recombinant retroviruses pseudotyped with either the gibbon ape leukemia virus (GaLV) envelope or the vesicular stomatitis virus G (VSV-G) glycoprotein. We show here that both retroviral particles mediate stable gene transfer in CD4+ and in CD8+ peripheral blood lymphocytes cultured under optimized conditions. However, VSV-G–pseudotyped virions may cause transduction artifacts that must be carefully excluded. The VSV-G virions require 10- to 100-fold higher concentrations of infectious particles to achieve levels of gene transfer comparable to GaLV-virions. Nonetheless, the physical stability of VSV-G–coated particles enables the concentration of viral stocks to 109 infectious particles per milliliter or more.
THE GENETIC alteration of primary T lymphocytes is essential for experimental and clinical investigation. Gene transfer mediated by murine leukemia viruses (MLV) is restricted by the expression of a suitable retroviral receptor1-3 and by cell cycle–dependent integration.4-6 Efficiency of gene transfer is also dependent on high-viral titers and optimized target cell culture conditions.7 We and others have observed that retroviral particles bearing the envelope of the Gibbon ape Leukemia Virus (GaLV) are more efficient than virions bearing the MLV amphotropic envelope for infecting human primary T lymphocytes.7-9 A recent report also suggested that virions bearing the G glycoprotein of vesicular stomatitis virus (VSV) as their envelope could efficiently mediate gene transfer in human T lymphocytes,10 although proviral integration was not examined. VSV-G–coated virions present a practical advantage in that they can be effectively concentrated by ultracentrifugation.11 Therefore, we compared the infectivity of both pseudotyped viral particles to establish optimal conditions for generating large populations of genetically modified peripheral blood lymphocytes (PBLs). We show here that particles pseudotyped with the GaLV envelope are far more infectious on a particulate basis, but that comparable gene transfer efficiency can be achieved with VSV-G–pseudotyped virions provided that they are highly concentrated and that transduction artifacts are excluded.
MATERIALS AND METHODS
Vector construction.A retroviral vector encoding the cell-surface marker NTP was constructed in the SFG vector backbone.12 The human low-affinity nerve growth factor (LNGFR) cDNA (kindly provided by Dr M. Chao, Cornell University Medical College, New York, NY) was mutated by linker insertion so as to abolish nerve growth factor (NGF) binding,13 and deleted in its 3′ end to create a molecule truncated at the plasma membrane that only retains 5 cytoplasmic amino acids (by insertion of the oligonucleotides CTGAGTCGACG and GATCCGTCGACTCAG). Thus, the molecule NTP is unable to bind to NGF and is disabled in its transduction function.13 14
Generation of high-titer producer cell lines and virus concentration.High-titer producer cells were generated by crossinfection of PG13 packaging cells15 with supernatant procured from the ecotropic BOSC packaging cell,16 or by cotransfection of 293GPG cells17 with vector DNA and pJ6Ωbleo plasmid DNA,18 as described by Riviere et al.19 Individual clones were expanded and tested for gene transfer under standardized conditions on either NIH 3T3, HeLa, or A549 (a human lung carcinoma) cells, all obtained from the American Tissue Type Collection (ATCC; Rockville, MD). Producer cells with the highest titers were identified by fluorescence-activated cell sorter (FACS) analysis and by Southern blot analysis as described.19,20 Virus concentration was performed as described.11
Viral titration.Viral titration was performed by infecting target cell lines with a serial dilution (1, 10−1, 10−2, 10−3) of the different viral stocks. Infections were carried out by applying 1 mL of supernatant or virus dilutions in tissue culture medium (DMEM [Cellgro, Herdon, VA], 10% calf serum, penicillin [50 U/mL], and streptomycin [50 μg/mL] for NIH 3T3; DMEM, 10% heat-inactivated fetal bovine serum, penicillin [50 U/mL], and streptomycin [50 μg/mL] for the other cell lines) supplemented with polybrene (Sigma, St Louis, MO) at a final concentration of 8 μg/mL. Infections were performed over 16 hours in 6-well plates (Costar, Cambridge, MA) for HeLa, A549, and human PBLs, or in 10-cm tissue culture dishes (in 3 mL) for the NIH 3T3 fibroblasts.19 The adherent cell lines were plated the day before infection, and one replicate well was obtained at the time of infection to measure the exact target cell number. After 72 to 96 hours, the cells were harvested, counted, stained with the anti-LNGFR monoclonal antibody 20.4 (ATCC), and analyzed by FACS analysis. The concentration of infectious particles was obtained by the following calculation: (% NTP+ cells) × (target cell number) × (1/dilution).
The fraction of positive cells and dilution used for this calculation was that measured under conditions that yielded between 50% and 10% positive cells. After harvesting the cells for FACS analysis, about half the cells were replated. Seven to 10 days after infection, genomic DNA was extracted for Southern blot analysis of vector copy number and structure.19 20
Detection of replication-competent retrovirus.Detection of replication-competent retrovirus (RCR) was carried out either by the hisD mobilization assay or by a modified Mus dunni assay. The mobilization assay, which is based on the ability of RCR to copackage a transcribed defective recombinant provirus encoding histidinol dehydrogenase expressed in a fibroblast cell line and to transmit the defective provirus to target cells (mobilization process), and the Mus dunni vector rescue assay are described elsewhere.17,19 21 The amphotropic 4070A wild-type virus obtained from a commercial source (Tektagen, Malvern, PA) served as a titrated positive control.
T-cell activation and retroviral infection.Peripheral blood mononuclear cells (PBMC) were separated on Ficoll and activated with phytohemagglutinin A (PHA) at 0.5 μg/mL (Murex Diagnostics, Norcross, GA) in a conventional medium consisting of RPMI 1640 medium (Cellgro) containing 10% heat-inactivated AB− pooled human serum (Pel Freez Biologicals, Brown Deer, WI) or fetal calf serum (Sigma). After 48 hours, one million cells were exposed to cell-free viral stocks filtered through a 0.45-μm filter or diluted VSV-G stocks for 18 hours in the presence of polybrene at 4 μg/mL and human recombinant interleukin-2 (rIL-2; Cetus, Emeryville, CA) at 10 U/mL. The next day, the cells were washed once and resuspended in medium containing 10% pooled human serum and rIL-2 at 10 U/mL. After 3 or 4 days, the cells were analyzed by FACS analysis or sorted in some experiments. After another week in culture, transgene expression was measured again by FACS analysis and genomic DNA was extracted for Southern blot analysis.
FACS analysis.For FACS analysis, cells were washed twice in phosphate-buffered saline, and stained with phycoerythrin-conjugated anti-CD3, -CD4, or -CD8 monoclonal antibody (Becton Dickinson, Mountain View, CA), and the anti-LNGFR antibody 20.4 (ATCC), which was detected by a FITC-labeled polyclonal goat antimouse antibody (Caltag, South San Francisco, CA). FITC-labeled antibodies against Vβ5, 6, 56, 8, and 12 were kindly provided by Dr D. Unutmaz (Skirball Institute, NYU Medical Center, New York, NY).
RESULTS
Vector construction.We constructed retroviral vectors encoding an inactive cell-surface marker that could serve to monitor gene expression and/or to purify transduced cells. The human LNGFR was modified as described in Materials and Methods to yield a mutated and truncated transmembrane protein unable to bind or respond to NGF.13,14 The variant, termed NTP, was cloned into the Moloney MLV-based vector SFG.12 High-titer producer cell lines were derived from the PG13 or 293GPG packaging cell lines and screened for helper virus, as described in Materials and Methods. No replication-competent retrovirus was detected in either the PG13/NTP6 or GPG/NTP7 clones.
Titration of GaLV and VSV-G retroviral stocks.The PG13/NTP6 supernatant was used as a cell-free viral stock while the GPG/NTP7 supernatant was concentrated from 1 L to 1 mL by ultracentrifugation. The GaLV envelope– and VSV-G glycoprotein–pseudotyped particles were titrated on standard cell lines as shown in Fig 1. The titers were 8 × 109 infectious particles per mL for the GPG/NTP7 concentrated stocks and 9 × 105 infectious particles per mL for the PG13/NTP6 supernatant, as measured on murine NIH 3T3 and human A549 cells, respectively. The titer of the VSV-G virions on HeLa and A549 cells was within a factor of 4 of that measured on NIH 3T3 (2 × 109 and 3 × 1010 infectious particles/mL, respectively). The measurements made by FACS analysis (Fig 1A) were confirmed by Southern blot analysis (Fig 1B), which demonstrated proviral integration and true gene transfer of the intact NTP vector without rearrangements.
Retroviral titration. NIH 3T3, HeLa, and A549 cells were plated the day before infection and cultured overnight with serial dilutions of the virus stocks, as described in Materials and Methods. The target cell numbers were counted at the beginning of the infection in one replicate well. (A) FACS analysis of cells 4 days after infection. The percentage of NTP+ cells is indicated in each quadrant. (B) Southern blot analysis of genomic DNA extracted from NIH 3T3 and A549 cells 7 days after infection. The DNA was digested with Nhe I and analyzed with a radiolabeled NTP probe. Samples corresponding to the 1, 10−1, 10−2, and 10−3 dilutions are loaded from left to right. On the left are two samples obtained from clones bearing one and two copies of the vector. EB, endogenous bands.
Retroviral titration. NIH 3T3, HeLa, and A549 cells were plated the day before infection and cultured overnight with serial dilutions of the virus stocks, as described in Materials and Methods. The target cell numbers were counted at the beginning of the infection in one replicate well. (A) FACS analysis of cells 4 days after infection. The percentage of NTP+ cells is indicated in each quadrant. (B) Southern blot analysis of genomic DNA extracted from NIH 3T3 and A549 cells 7 days after infection. The DNA was digested with Nhe I and analyzed with a radiolabeled NTP probe. Samples corresponding to the 1, 10−1, 10−2, and 10−3 dilutions are loaded from left to right. On the left are two samples obtained from clones bearing one and two copies of the vector. EB, endogenous bands.
Gene transfer in human primary T lymphocytes using GALV-pseudotyped virions.PBMC freshly harvested from healthy donors were cultured in the presence of PHA for 48 hours before exposure to retroviral particles. The cells were stained 3, 10, and 16 days after infection with anti–T-cell marker and anti-LNGFR monoclonal antibodies. Overnight cocultivation of one million preactivated PBMC with irradiated PG13/NTP6 producer cells resulted in 25% to 35% NTP+ cells among the CD3+ cells (data not shown). Infection with cell-free viral stocks obtained from the same producer cell consistently yielded 22% to 28% positive cells in over 10 experiments with PBMC from different donors (Fig 2A and B). These analyses confirmed that LNGFR is not expressed in quiescent or activated T lymphocytes,7,9,22,23 and established that CD3+ cells represented 95% or more of the cells present in the cultures. A comparable fraction of both CD4+ and CD8+ subsets were NTP-positive (Fig 2C and D). The same percentage was measured in Vβ5, 6, 56, 8, and 12-positive lymphocytes (data not shown), suggesting that the infection did not preferentially target a subset of the T-cell repertoire and corroborating the findings of Rudoll et al9 in CD4+ lymphocytes. Southern blot analysis performed on genomic DNA extracted from sorted CD3+NTP+ cells 4 days after infection with the GaLV virions showed unrearranged vector sequences of the predicted size at an average of 1 vector copy per cell (Fig 3).
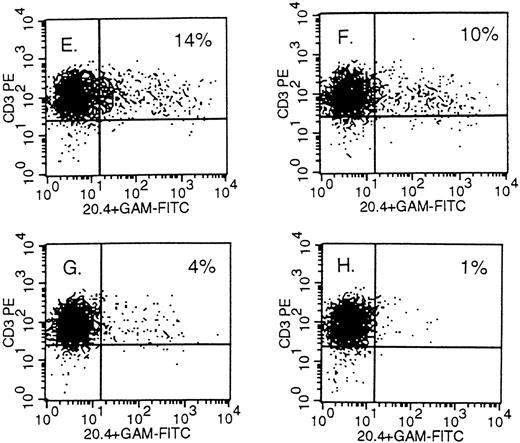
Detection of NTP expression by FACS analysis in retrovirally transduced human primary T lymphocytes. On the X-axis, staining of the mutated LNGFR; on the Y-axis, staining of CD3 in (A), (B) and (E) through (H); CD4 in (C); and CD8 in (D). The PBMC were infected 10 days earlier with PG13 supernatant in (A) and with PG13/NTP6 supernatant in (B) through (D). In (E) through (H), the PBMC were infected with a 1:300, 1:3,000, 1:30,000, and 1:300,000 dilution of the concentrated 293gpg/NTP7 stock, respectively, and stained 10 days thereafter. The percentage indicated in the upper right quadrant is the percentage of NTP+ cells among the CD3+, CD4+, or CD8+ cells.
Detection of NTP expression by FACS analysis in retrovirally transduced human primary T lymphocytes. On the X-axis, staining of the mutated LNGFR; on the Y-axis, staining of CD3 in (A), (B) and (E) through (H); CD4 in (C); and CD8 in (D). The PBMC were infected 10 days earlier with PG13 supernatant in (A) and with PG13/NTP6 supernatant in (B) through (D). In (E) through (H), the PBMC were infected with a 1:300, 1:3,000, 1:30,000, and 1:300,000 dilution of the concentrated 293gpg/NTP7 stock, respectively, and stained 10 days thereafter. The percentage indicated in the upper right quadrant is the percentage of NTP+ cells among the CD3+, CD4+, or CD8+ cells.
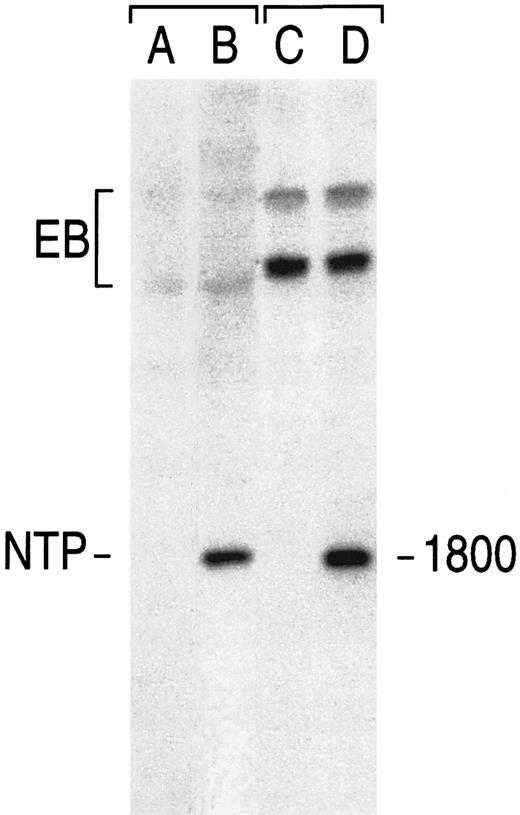
Detection by Southern blot analysis of a vector sequence in sorted human primary T lymphocytes. Cells infected with GaLV-virions bearing the NTP vector were sorted based on binding of the 20.4 monoclonal antibody. See text for transduction and staining conditions. Genomic DNA was digested with Nhe I. Blots were probed with radiolabeled NTP cDNA. In control lanes (left), the signal corresponds to 1 vector copy per cell in NIH 3T3 fibroblasts (A, noninfected NIH 3T3; B, NTP). In the right lanes, the signal found in noninfected human T lymphocytes (C) and in sorted NTP+ lymphocytes (D). The endogenous bands (EB), which differ between mouse and human DNA, show the even loading of each sample.
Detection by Southern blot analysis of a vector sequence in sorted human primary T lymphocytes. Cells infected with GaLV-virions bearing the NTP vector were sorted based on binding of the 20.4 monoclonal antibody. See text for transduction and staining conditions. Genomic DNA was digested with Nhe I. Blots were probed with radiolabeled NTP cDNA. In control lanes (left), the signal corresponds to 1 vector copy per cell in NIH 3T3 fibroblasts (A, noninfected NIH 3T3; B, NTP). In the right lanes, the signal found in noninfected human T lymphocytes (C) and in sorted NTP+ lymphocytes (D). The endogenous bands (EB), which differ between mouse and human DNA, show the even loading of each sample.
Gene transfer in human primary T lymphocytes using VSV-G–pseudotyped virions.Mitogen-stimulated PBMC were infected with serially diluted VSV-G–pseudotyped particles under the conditions described above for GaLV-pseudotyped virions. One million T cells were exposed in 1 mL of culture medium to dilutions of the concentrated stock of 1:300, 1:3,000, 1:30,000, and 1:300,000, corresponding to a multiplicity of infection (m.o.i.) of 100, 10, 1, and 0.1, respectively, based on the infectious viral titer measured in A549 cells (Fig 1). As shown in panels E through H of Fig 2, 14%, 10%, 4%, and 1% of the CD3+ cells were NTP+ when stained 10 days after infection against a background of 1%. As opposed to what we found with the GaLV virions, gene transfer using concentrated VSV-G virions cannot be inferred from FACS analysis 2 to 4 days after infection. Using VSV-G virions, we repeatedly observed that the fraction of positive cells decreased over the first 5 days in culture and remained stable during days 10 through 16. On day 3, the samples shown in Fig 2, panels E through H, were 86%, 61%, 21%, and 6% NTP+ by FACS analysis, respectively. As shown in the upper section of Fig 4, NTP+ cells represent 83% and 12% of the CD3+ cells on day 4 and 10, respectively. The cells were sorted on day 4 into three fractions (NTP−, NTPlo, and NTPhi; see middle section of Fig 4), and examined by Southern blot analysis for proviral integration. As shown in the lower panel, the NTPlo fraction was largely comprised of nontransduced cells whereas the NTPhi fraction contained mostly transduced cells. On day 10, the FACS profile of the infected populations invariably resolved into two distinct subsets, corresponding to NTP− and NTP+ cells, as shown in Fig 4, top. On day 10, the positive cells showed proviral integration by Southern blot analysis at an average of 1 copy per cell, as shown for the NTPhi fraction in Fig 4.
Gene transfer in primary T lymphocytes infected with VSV-G–coated virions. (Upper panel) One million lymphocytes were infected with VSV-G virions at a multiplicity of infection of 100 as described in Fig 2. Cells were analyzed for NTP expression 3 days after infection (left panel) and 10 days after infection (right panel). (Middle panel) CD3+ cells were sorted on day 4 in three groups: NTP− cells (N), NTPlo cells (L), and NTPhi cells (H). (Lower panel) Southern blot analysis of the three sorted fractions. See Figs 1 and 3 for assay conditions and copy number controls. The N fraction shows virtually no vector signal. The L fraction shows a faint band, indicating that a majority of the cells in this fraction do not have integrated viral sequences. The H fraction shows a strong signal, corresponding to about 1 vector copy per cell.
Gene transfer in primary T lymphocytes infected with VSV-G–coated virions. (Upper panel) One million lymphocytes were infected with VSV-G virions at a multiplicity of infection of 100 as described in Fig 2. Cells were analyzed for NTP expression 3 days after infection (left panel) and 10 days after infection (right panel). (Middle panel) CD3+ cells were sorted on day 4 in three groups: NTP− cells (N), NTPlo cells (L), and NTPhi cells (H). (Lower panel) Southern blot analysis of the three sorted fractions. See Figs 1 and 3 for assay conditions and copy number controls. The N fraction shows virtually no vector signal. The L fraction shows a faint band, indicating that a majority of the cells in this fraction do not have integrated viral sequences. The H fraction shows a strong signal, corresponding to about 1 vector copy per cell.
Comparison of infectivity of GaLV– and VSV-G–pseudotyped virions.NTP expression remained stable between days 3 and 16 in lymphocytes infected with the GaLV-pseudotyped virions, in contrast to the dynamic profile observed in VSV-G–infected lymphocytes. A summary of stable gene transfer efficiencies obtained at different viral concentrations for both GaLV– and VSV-G–coated particles is shown in Table 1. When the viral concentrations are normalized by titrating both stocks on the same cell line (A549, Fig 1), we observed that the GaLV virions are more efficient on a particulate basis. The VSV-G virions diluted in the same culture medium required 10- to 100-fold higher concentrations of infectious particles to achieve levels of gene transfer comparable to GaLV-virions (Table 1). Thus, both types of virions eventually yielded comparable levels of CD3+NTP+ cells after culture under identical conditions, provided that greater concentrations of the VSV-G virions were used.
DISCUSSION
The efficient generation of primary lymphocytes is of great interest to the study of T-cell function in vitro and the manipulation of the immune response in vivo. One essential premise in current transduction strategies is to maximize gene transfer efficiency and minimize the duration of cell culture. The selection of infected and transfected lymphocytes typically requires 10 to 20 days when conventional drug resistance is used. Prolonged lymphocyte culture can be avoided by the use of cytoplasmic or cell-surface markers that are suitable for rapid purification procedures. Using a reliable vector design, cell-surface markers provide the means to accurately quantify, rapidly purify, and continuously monitor the cells that express the transgene. The criteria for selecting a marker suitable for human studies are that the reporter be absent in normal T cells; inactive in T cells; nonimmunogenic, ie, human and nonpolymorphic; readily detectable by a good monoclonal antibody; and that its cDNA be small and not adversely affect viral titers. We describe here a novel mutant of the human LNGFR, a marker suggested earlier by Mavilio et al,22 which we modified to meet criteria required for in vivo studies. In NTP, a mutation in the NGF binding site is combined with a cytoplasmic truncation, both of which are necessary to generate an inactive molecule.14 The NTP marker is unlikely to be antigenic in vivo on the basis of the point mutation in its NGF binding site, although its immunogenicity cannot be ruled out.
We show here that particles enveloped with either the GaLV envelope or the VSV-G glycoprotein mediate stable and efficient gene transfer in human primary CD4+ and CD8+ T lymphocytes. Stable gene transfer was confirmed by Southern blot analyses (Figs 3 and 4), demonstrating integration of the intact proviral structure. Gene transfer is proportional to the concentration of infectious particles (Figs 1 and 2 and Table 1). However, the utilization of concentrated VSV-G–coated virions could lead to artifacts when gene transfer was indirectly assessed by immunofluorescence in the first 5 days after infection (Fig 4). Cell sorting at early time points will yield a mix of transduced and nontransduced cells. The high frequency of false-positive cells — cells positive by FACS analysis but negative by Southern blot analysis — commands caution when gene transfer is solely inferred from cell surface analysis at early time points and not supported by DNA analyses.10,24 The mechanism underlying this pseudotransduction remains unclear, but may relate to the particular envelope structure of VSV-G–pseudotyped virions25 or perhaps some form of protein transfer.26
To compare the relative infectivity of the two types of particles, the viral stocks were titrated on the same cell line (Fig 1). Thus, the multiplicity of infection shown in Fig 2 and Table 1 is based on titrations obtained in A549 cells. The titrations vary, depending on which cell line is used as an indicator cell line, and are fourfold less when measured on NIH 3T3 cells rather than on A549 cells (Fig 1). GaLV particles bind to the GLVR-1 receptor,4-6 whereas the receptor for VSV-G is a ubiquitous membrane glycolipid27 expressed in various mammalian and nonmammalian cells.26,28-30 The infectivity of VSV-G virions varies greatly among different cells of hematopoietic origin (unpublished observations) and is 10-fold less efficient than amphotropic particles for the infection of hepatocytes.26 Nonetheless, the physical stability of VSV-G–coated particles enables the generation of viral stocks containing more than 109 infectious particles per milliliter17,31,32 and the stable conservation of frozen viral stocks. Concentrated stocks are attractive in that they can be added as a small volume to any medium. The generation of viral stocks is also facilitated through the use of 293-based transient packaging cell lines.17 32 In contrast, the GaLV-coated particles can be used as viral supernatants without necessitating the generation of a large volume of culture supernatant followed by virus concentration.
ACKNOWLEDGMENT
We thank K. Srivastava and T. Delohery for excellent assistance with virus concentration and cell sorting, respectively; V. Petronis and A. Krause for excellent assistance with preparation of the manuscript and artwork; and I. Rivière for reviewing the manuscript.
Supported in part by Grant No. CA59350. M.S. is a recipient of the McDonnell Foundation Scholars Award.
Address reprint requests to M. Sadelain, MD, PhD, Box 182, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021.