Abstract
Human T-cell leukemia virus type-I (HTLV-I), the etiologic agent of adult T-cell leukemia (ATL) transforms human T cells both in vivo and in vitro. However, the long latency period between infection and development of ATL, as well as the small fraction of the infected population that actually develops this disease, suggest that factors in addition to the virus are involved in its pathogenesis. Mutation of tumor suppressor gene p53 has been found in both HTLV-I–transformed T-cell lines and ATL cases at relatively low frequency. However, increasing evidence supports p53 functional impairment in HTLV-I–transformed T cells. Tax, the major transactivator of HTLV-I, is critical for the initial events involved in transformation. We have considered the possibility that p53 may regulate transcription of viral and cellular genes important for viral replication and transformation. Inactivation of p53 function might then permit constitutive expression of these viral and cellular genes. We have investigated the effects of wild-type and mutant p53 on Tax-mediated activation of the HTLV-I long terminal repeat (LTR) and the promoters of several cellular genes including the interleukin (IL)-1α, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF ), and IL-2 receptor α chain gene. Jurkat, HuT78, and U937 cells were cotransfected with plasmids containing a chloramphenicol acetyltransferase (CAT ) reporter gene under viral or cellular promoter control and the Tax expression vector, in addition to vectors for a wild-type or mutant p53. Wild-type p53 is a potent repressor of viral and cellular activation by Tax. Mutations within p53 severely inhibit this downregulation. We also show that wild-type p53 suppresses transcription from the HTLV-I LTR in Jurkat-Tax, a T-cell line stably expressing Tax, and MT-2, a HTLV-I–transformed T-cell line. Wild-type, but not mutant, p53 interfered with the binding of TATA-binding protein (TBP) to the TATA motif of the HTLV-I LTR. These results suggest that p53 inactivation may lead to upregulation of viral and cellular genes and may also be important for establishment of productive viral infection and development of ATL.
ADULT T-CELL LEUKEMIA (ATL) is a highly aggressive T-cell malignancy etiologically linked to a retrovirus, human T-cell leukemia virus type-I (HTLV-I).1,2 Transformation by HTLV-I both in vitro and in vivo occurs in a slow stepwise manner,3,4 and progresses from oligoclonality to monoclonal dominance with decreased interleukin-2 (IL-2) dependence.5 The existence of host suppressors that inactivate HTLV-I is proposed because of the long latency period for development of ATL, usually greater than 50 years, with only 2% to 5% of infected individuals developing leukemia.6 The HTLV-I genome does not possess typical proto-oncogenic sequences,7 nor does it activate cellular proto-oncogenes by integration at specific genomic sites.8 Karyotypic analysis of lymphocytes from ATL patients shows no consistent structural abnormality. Therefore, other factors in addition to HTLV-I infection appear to be involved in the development of ATL. Multistep carcinogenesis for ATL is suggested by stochastic analysis9; however, none of the proposed additional events have been identified.
A potential candidate gene involved in the evolution to a malignant phenotype is the p53 tumor suppressor gene. p53 plays an important role in cell-cycle regulation and DNA repair and is often deleted and mutated in a wide variety of human malignancies.10 Recent studies have shown mutations in the highly conserved regions of the gene in only a minority of HTLV-I–transformed T-cell lines and in one fourth of ATL samples.11-14 Since p53 mutations are not found in all patients, the possibility that inactivation of p53 could occur by alternate mechanisms is suggested. Viral proteins of DNA-transforming viruses, such as SV40 and adenovirus, can inactivate p53 function through direct binding.15,16 Wild-type p53 protein is usually detected at low steady-state levels, due to the rapid turnover of newly synthesized protein.17-19 However, the patterns of expression of mutant p53 and wild-type p53 complexed with SV40 T-antigen or adenovirus 5 E1B differ from that of wild-type p53. Both mechanisms can prolong the half-life of the p53 protein. Some studies have demonstrated elevated steady-state levels of p53 protein in the majority of HTLV-I–transformed cell lines despite the presence of the wild-type sequence in the p53 coding region compared with nontransformed lymphocytes, suggesting functional impairment of the protein.20-22 The mechanism responsible for the increased stabilization of p53 in these cells is presently unknown. Additionally, a recent report has observed elevated levels of p53 in IL-2–independent cell lines relative to IL-2–dependent lines.23 In fact, several lines of evidence suggest the functional inactivation of p53 in HTLV-I–transformed T cells.23 24
HTLV-I encodes a 40-kd protein, Tax, which transforms rodent fibroblasts,25 immortalizes normal human T cells,26 and can induce a leukemia-like disease in transgenic mice.27 Tax is critical for HTLV-I gene regulation28-30 and has been shown to modulate the expression of a number of cellular genes critical for proliferation or growth processes, such as the genes encoding IL-2, IL-2 receptor (IL-2R), IL-1, IL-6, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF ), c-Fos, Fra-1, c-Jun, JunB, and JunD.31-37 The deregulation of these Tax-responsive cellular genes may play an important role in HTLV-I transformation. Thus, Tax appears to be necessary but not sufficient for malignant transformation, as only a small percentage of infected individuals develop ATL following a latency period of several decades.
The p53 protein is a transcription factor that can bind specifically to DNA sequences in various promoters and stimulate their transcriptional activity.38 It can also function as a transcriptional repressor of many growth factor–regulated genes.39,40 Interestingly, Uittenbogaard et al41 demonstrated transcriptional repression of p53 by HTLV-I Tax.41 However, the converse effect of p53 on the promoter activity of HTLV-I has not been investigated. Consequently, we were interested in determining whether p53 inactivation could upregulate the HTLV-I long terminal repeat (LTR) and cellular promoters, leading to the overexpression of HTLV-I and several cellular genes (cytokines and their receptors) seen in HTLV-I–transformed cells.
We studied the effect of wild-type and mutant human p53 expression on the activity of HTLV-I and several cellular promoters fused to a chloramphenicol acetyltransferase (CAT) reporter gene. We found that wild-type p53 is a potent repressor of Tax transactivation of the HTLV-I LTR and several cellular promoters.
MATERIALS AND METHODS
Cell lines. The human T-cell acute lymphocytic leukemia line Jurkat, the cutaneous T-cell lymphoma line HuT78,42 the HTLV-I–infected T-cell line MT-2,43 and the monocytoid cell line U937 were maintained in RPMI 1640 medium (GIBCO BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, 50 U/mL penicillin, 50 μg/mL streptomycin, and L-glutamine at 37°C and 5% CO2 . Jurkat-Tax cells were derived from Jurkat cells that were stably transfected with the HTLV-I Tax gene driven by the promoter for human cytomegalovirus (HCMV).35 Expression of Tax was confirmed by reverse transcription–polymerase chain reaction and by transactivation of the CAT gene driven by the HTLV-I LTR. Jurkat-Tax cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 400 μg G418 (Life Technologies, Gaithersburg, MD) per milliliter.
DNA plasmids. The CAT plasmids described herein all contain the Escherichia coli CAT gene under transcriptional control of the following promoters: HTLV-I LTR,32 αH2 (human IL-1α promoter),32 IL-6 (human IL-6 promoter),44 GM-CSF (human GM-CSF promoter),36 and IL-2R (human IL-2R α chain promoter).32 BC3.9 Sph is a Tax expression vector driven by the HCMV promoter.32 The negative control BC12 is the backbone plasmid for BC3.9 Sph. The plasmid containing the wild-type p53 cDNA (pC53-SN3), plasmids containing mutant p53 cDNAs (pC53-SCX3, pC53-248W, and pC53-273H) cloned from human colorectal carcinoma cell lines,45 and the p53 control vectors PG13-CAT and MG15-CAT46 were kindly provided by Dr B. Vogelstein. PG13-CAT has 13 head-to-tail copies of a wild-type p53-specific binding DNA sequence (5′-CCTGCCTGGACTTGCCTGG-3′). MG15-CAT has 15 head-to-tail copies of a mutated sequence (5′-CCTTAATGGACTTTAATGG-3′). All of the p53 cDNAs are cloned in the BamHI site of the neomycin-resistant gene-carrying plasmid pCMV-NeoBam45 downstream of the HCMV early promoter. pC53-SCX3 (143A), pC53-248W (248W), and pC53-273H (273H) have point mutations at codon 143 (Val to Ala), codon 248 (Arg to Trp), and codon 273 (Arg to His), respectively. All plasmids were prepared with Qiagen columns (Qiagen, Chatsworth, CA) according to the directions of the manufacturer.
Transient transfections and CAT assay. Plasmids were transfected by electroporation using a Bio-Rad Gene Pulser (Bio-Rad, Richmond, CA) at 960 μF and 250 V as described previously.32 The amount of plasmid used for one transfection was 5 μg for HTLV-I LTR CAT and 10 μg for αH2, IL-6 CAT, GM-CSF CAT, and IL-2R CAT, and various amounts of p53 expression plasmids as indicated in the figures. Transfections were repeated at least three times per experimental variable, and for each one the total amount of plasmid remained constant. After 48 hours, cells were harvested and whole-cell extracts were assayed for CAT activity by a thin-layer chromatography method described previously.32
Electrophoretic mobility shift assay. Recombinant TATA box–binding protein (TBP) was purchased from Promega (Madison, WI) and used at a concentration of 1 footprint unit (FPU) per reaction. Electrophoretic mobility shift assay (EMSA) was performed by preincubating TBP, GST-p53 wild-type protein, codon 135 mutant GST-p53 protein, or a combination of these proteins in 10 μL buffer containing 10% glycerol, 20 mmol/L Tris-HCl, pH 8.0, 80 mmol/L KCl, 10 mmol/L MgCl2 , and 2 mmol/L dithiothreitol in the presence or absence of the indicated unlabeled DNA competitor at room temperature. After 10 minutes, 50,000 cpm of the labeled fragment was added and the reaction was incubated for an additional 15 minutes. The probe, 5′-tcgaCTAGCAGGAGTCTATAAAAGCGTGGAGACAG-3′, was a double-stranded oligonucleotide corresponding to positions −40 to −10 of the HTLV-I promoter. In the case of mutant competitor, the TATA box (underlined in the above sequence) was replaced by AGAAC. The sequences of a TBP consensus competitor and an NF-κB oligonucleotide were 5′-tcgaGCAGAGCATATAAGGTGAGGTAGGA-3′ and 5′-gatcACAAGGGACTTTCCGCTGGGGACTTTCCAG-3′, respectively. The samples were analyzed on a 6% native polyacrylamide gel pre-electrophoresed for 20 minutes at 300 V, using 0.5× TBE (1× TBE is 50 mmol/L Tris, 50 mmol/L boric acid, and 2 mmol/L EDTA) with 4 mmol/L MgCl2 and 0.02% of Nonidet P-40 as running buffer.
RESULTS
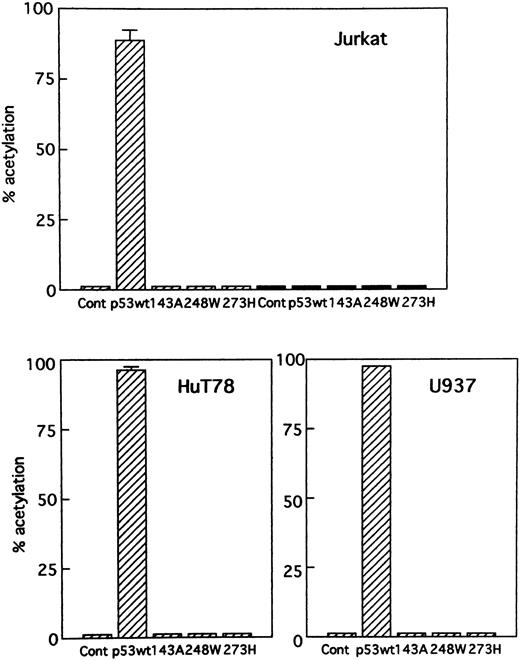
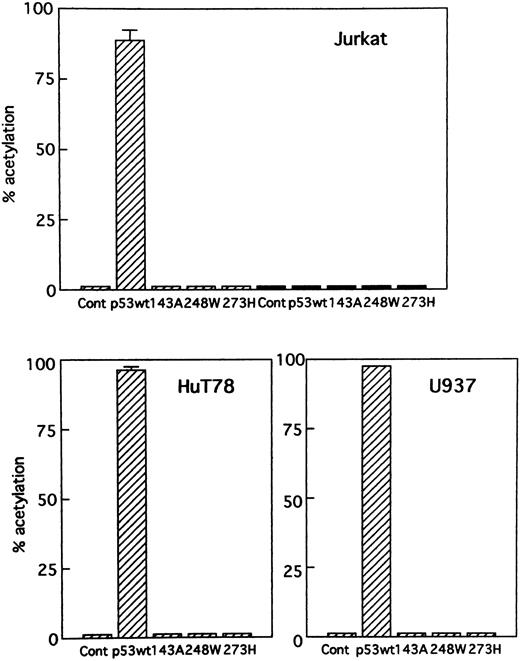
Activation of gene expression by p53. To determine if p53 expression vectors were functioning properly in Jurkat, HuT78, and U937 cells, the effect of the wild-type and mutant p53 expression vectors was first studied on p53 control constructs. These reporter gene constructs contain multiple copies of either a consensus p53 binding sequence for the positive control PG13-CAT or a scrambled p53 binding sequence for the negative control MG15-CAT adjacent to a minimal promoter and CAT reporter gene. Figure 1 shows that the wild-type p53 expression vector stimulated the p53-positive control PG13-CAT and failed to stimulate the p53-negative control MG15-CAT in these cells. The mutants of p53 chosen for this study were already described and are as follows: pC53-SCX3 (143A), pC53-248W (248W), and pC53-273H (273H). The mutant p53 expression vectors did not stimulate either control vector. Thus, wild-type and mutant p53 expression vectors behaved as expected in an assay with p53 control reporter constructs. No mutations were found in the p53 coding region in the HTLV-I–infected cell line MT-2.11,14,23,24 The plasmid PG13-CAT was used to determine the relative levels of functional p53 protein in MT-2. In accordance with the previous experiments,23 MT-2 and Jurkat, HuT78, and U937 cells had no measurable CAT activity (data not shown).
Transient expression of p53 control constructs. Cells were cotransfected with (▨) PG13-CAT, a wild-type p53–inducible positive control construct (10 μg), or (▪) MG15-CAT, a negative control construct (10 μg), and wild-type or several mutant p53 expression vectors (5 μg).
Transient expression of p53 control constructs. Cells were cotransfected with (▨) PG13-CAT, a wild-type p53–inducible positive control construct (10 μg), or (▪) MG15-CAT, a negative control construct (10 μg), and wild-type or several mutant p53 expression vectors (5 μg).
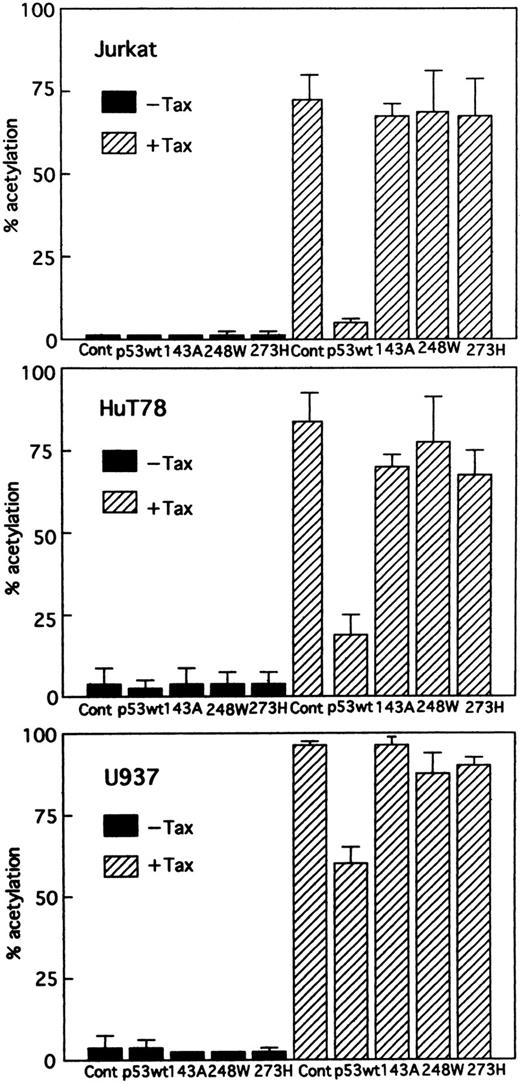
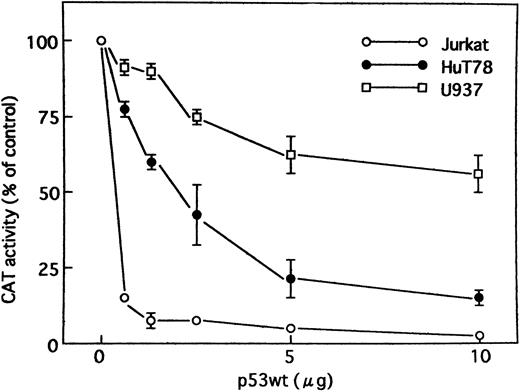
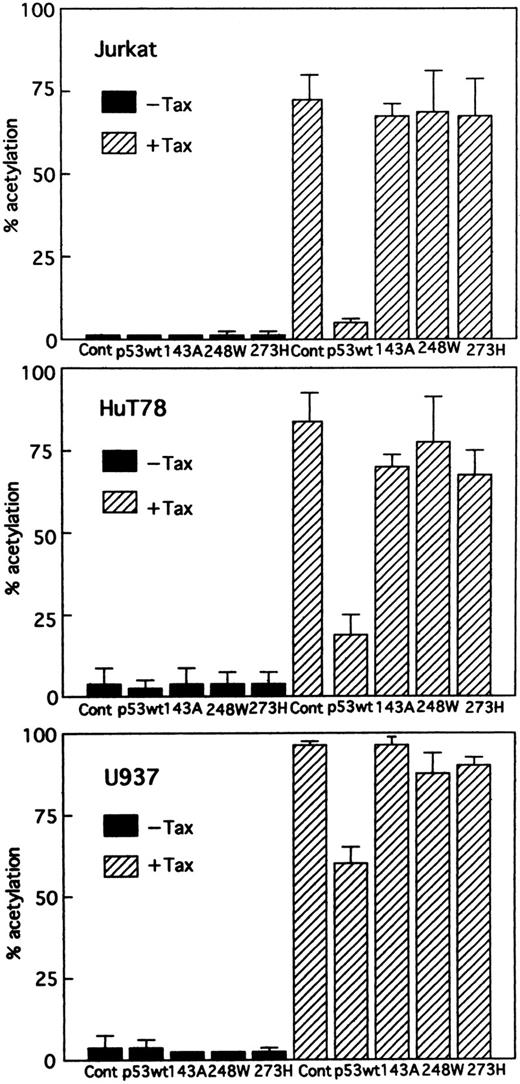
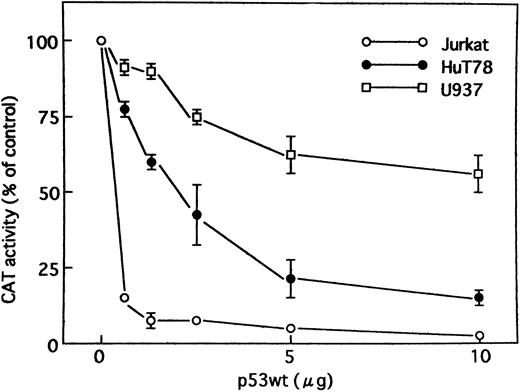
Wild-type p53 suppresses transactivation of HTLV-I LTR by Tax. Tax promotes viral replication resulting in random infection of T cells and also promotes proliferation of infected T cells. We were interested in determining whether wild-type p53 can inhibit activation of the HTLV-I LTR by Tax and, if so, whether mutants of p53 exert the same effect. Jurkat, HuT78, and U937 cells were cotransfected with the HTLV-I LTR–driven CAT reporter construct and a Tax expression vector (BC3.9 Sph) or the parental vector BC12, in addition to an expression construct for the human wild-type or various mutants of p53 or the parental vector pCMV-NeoBam. The amount of Tax expression vector transfected (5 μg) was sufficient for maximal stimulation of the HTLV-I LTR reporter gene. The cells used in this study are null for p53. They have no detectable endogenous p53 protein by Western blot analysis, which could influence the transfection results.47,48 Direct sequencing of the endogenous genomic p53 revealed a homozygous or heterozygous mutation at codon 196 (CGA → CGA/TGA) in Jurkat cells47,49 and a homozygous mutation at codon 196 (CGA → TGA) in HuT78 cells.49 U937 cells have point mutations that convert G to A at the first base of intron 5.50 The majority of these mutations occur in the central domain of the p53 molecule, which is the region involved in DNA binding. After 48 hours, CAT activity (HTLV-I LTR activity) was assayed. Wild-type p53 inhibited Tax-mediated activation of HTLV-I LTR CAT in transient assays by greater than 14-fold in Jurkat and fourfold in HuT78 cells, respectively, whereas the mutant p53s had a relatively minor effect, if any, on expression of the HTLV-I LTR CAT construct (Fig 2). Similar results were also observed when using U937 cells, although less inhibition was seen (Fig 2). To ensure that the transfection efficiency in all cell lines was comparable, cells were transfected with a CMV-CAT vector. The levels of CAT generated by this plasmid in all cell lines were almost similar. Thus, the differing effects in different cell lines do not reside in their differences in transfection efficiency. In addition, we cotransfected increasing amounts of an expression vector for wild-type p53. The parental vector (pCMV-NeoBam) was included to normalize the amount of transfected DNA. Figure 3 shows that a dose-dependent decrease in Tax-mediated promoter activity was observed. To test whether this inhibition is a secondary consequence of growth arrest by p53, we assayed the growth of Jurkat cells: wild-type p53 did not suppress growth (data not shown). Thus, the promoter inhibition is due to wild-type p53, and mutations in the p53 gene abolish the inhibitory effect.
Effect of wild-type and different mutant p53s on stimulation of the HTLV-I promoter by Tax. Jurkat, HuT78, and U937 cells were cotransfected with HTLV-I LTR CAT (5 μg) and 5 μg Tax expression vector (BC3.9 Sph, + Tax) or the parental vector (BC12, –Tax), together with 5 μg of the vector control plasmid (pCMV-NeoBam), or the expression construct of either wild-type or one of the mutant p53s: 143A (V to A at amino acid 143), 248W (R to W at amino acid 248), and 273H (R to H at amino acid 273). Columns, mean % acetylation from ≥3 independent transfections; bars, SEM.
Effect of wild-type and different mutant p53s on stimulation of the HTLV-I promoter by Tax. Jurkat, HuT78, and U937 cells were cotransfected with HTLV-I LTR CAT (5 μg) and 5 μg Tax expression vector (BC3.9 Sph, + Tax) or the parental vector (BC12, –Tax), together with 5 μg of the vector control plasmid (pCMV-NeoBam), or the expression construct of either wild-type or one of the mutant p53s: 143A (V to A at amino acid 143), 248W (R to W at amino acid 248), and 273H (R to H at amino acid 273). Columns, mean % acetylation from ≥3 independent transfections; bars, SEM.
Tax activity was suppressed by wild-type p53 when expression constructs for Tax and wild-type p53 were cotransfected. Jurkat, HuT78, and U937 cells were transiently cotransfected with HTLV-I LTR CAT reporter construct (5 μg) and a Tax expression vector (5 μg), together with increasing amounts of an expression construct encoding human wild-type p53 protein. The control vector pCMV-NeoBam was used to normalize the amount of expression vector in each transfection. Values for CAT activity are expressed as a percentage of levels seen in the absence of p53. Experiments were performed ≥3 times. Where not shown, SEM bars are smaller than the symbol size.
Tax activity was suppressed by wild-type p53 when expression constructs for Tax and wild-type p53 were cotransfected. Jurkat, HuT78, and U937 cells were transiently cotransfected with HTLV-I LTR CAT reporter construct (5 μg) and a Tax expression vector (5 μg), together with increasing amounts of an expression construct encoding human wild-type p53 protein. The control vector pCMV-NeoBam was used to normalize the amount of expression vector in each transfection. Values for CAT activity are expressed as a percentage of levels seen in the absence of p53. Experiments were performed ≥3 times. Where not shown, SEM bars are smaller than the symbol size.
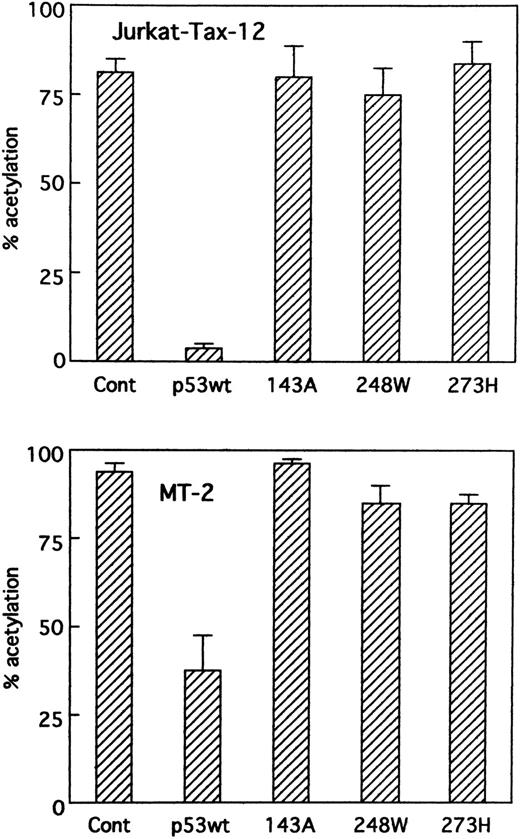
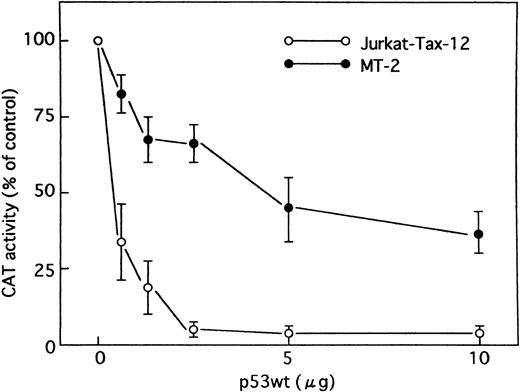
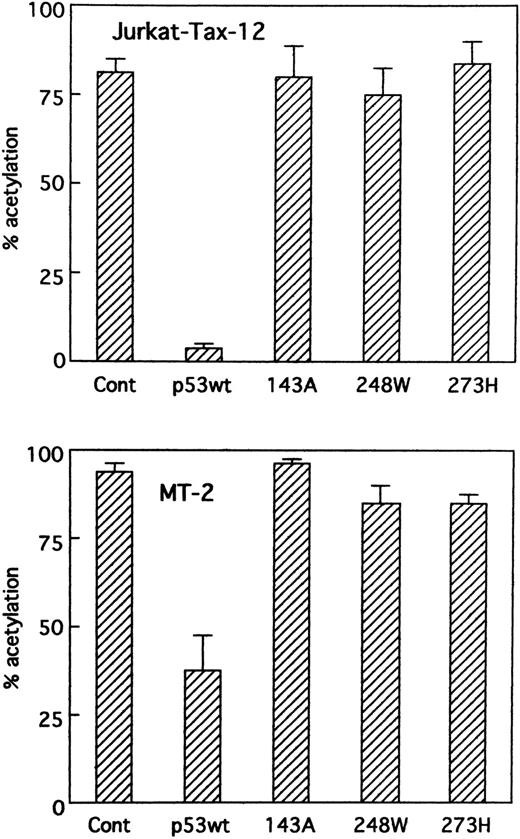
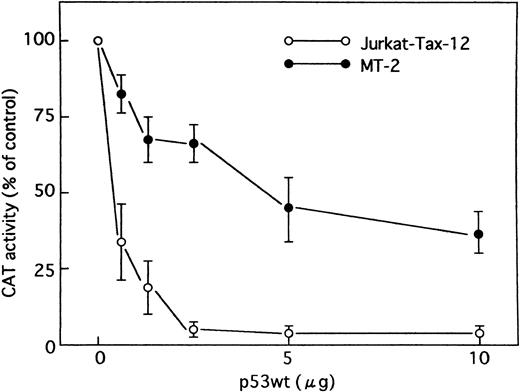
Wild-type p53 in Jurkat cells stably transfected with the HTLV-I Tax (Jurkat-Tax)35 inhibited transactivation of HTLV-I LTR by Tax in a dose-dependent manner (Figs 4 and 5). On the other hand, mutant versions of the p53 protein were unable to repress transcription (Fig 4). Our results were further confirmed using different clones of Jurkat-Tax to rule out tissue culture artifacts and also, importantly, to demonstrate reproducibility of the results (data not shown). Similar results were also observed in a Tax-expressing HTLV-I–infected T-cell line, MT-2, which lacks functional p53 activity,23 although less inhibition by wild-type p53 was noted (Figs 4 and 5). Transfection efficiencies in these two cell lines were also almost similar.
Suppression of Tax activity by wild-type p53 in cells containing Tax protein. Jurkat-Tax cells that constitutively express Tax and MT-2 cells were transiently transfected with HTLV-I LTR CAT reporter construct (5 μg) and expression vectors for wild-type p53, mutant p53s (143A, 248W, and 273H), or parental vector pCMV-NeoBam (5 μg). CAT activity was measured on cellular extracts prepared 48 hours after transfection.
Suppression of Tax activity by wild-type p53 in cells containing Tax protein. Jurkat-Tax cells that constitutively express Tax and MT-2 cells were transiently transfected with HTLV-I LTR CAT reporter construct (5 μg) and expression vectors for wild-type p53, mutant p53s (143A, 248W, and 273H), or parental vector pCMV-NeoBam (5 μg). CAT activity was measured on cellular extracts prepared 48 hours after transfection.
Effect of p53 on HTLV-I promoter in cells containing Tax protein. Jurkat-Tax and MT-2 cells were transfected with HTLV-I LTR CAT reporter construct (5 μg), together with increasing amounts of an expression vector encoding wild-type p53 as indicated. In all cases, the total amount of DNA transfected was maintained at 15 μg by addition of control plasmid (pCMV-NeoBam). Values for CAT activity are expressed as a percentage of levels seen in the absence of p53. Data are the mean ± SEM for 3 independent transfections.
Effect of p53 on HTLV-I promoter in cells containing Tax protein. Jurkat-Tax and MT-2 cells were transfected with HTLV-I LTR CAT reporter construct (5 μg), together with increasing amounts of an expression vector encoding wild-type p53 as indicated. In all cases, the total amount of DNA transfected was maintained at 15 μg by addition of control plasmid (pCMV-NeoBam). Values for CAT activity are expressed as a percentage of levels seen in the absence of p53. Data are the mean ± SEM for 3 independent transfections.
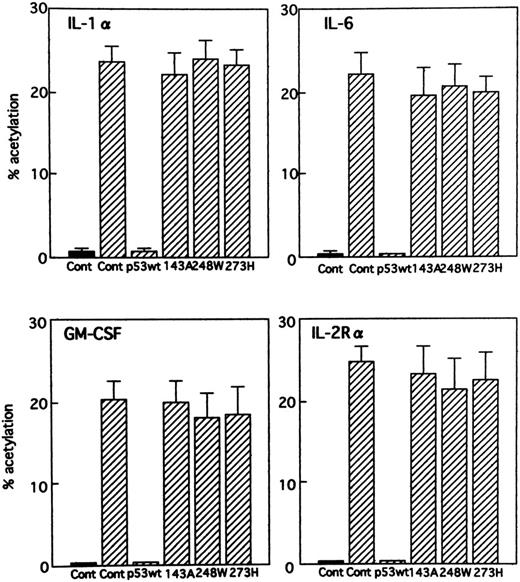
Effect of expression of wild-type and various mutants of p53 on several cellular gene promoters. Tax transactivates the transcription of the viral genome and also of many cellular genes. An appealing explanation for overexpression of cellular genes including cytokines and their receptors in ATL would be that the inactivation of p53 might allow for constitutive activation of these genes. This would suggest that the genes are normally repressed by wild-type p53. To analyze the effect of wild-type and various mutants of p53 on several cellular promoters, we used the following promoter-CAT constructs: IL-1α (αH2), IL-6 promoter (IL-6 CAT), GM-CSF promoter (GM-CSF CAT), and IL-2R α chain promoter (IL-2R CAT). The promoter activities were determined by CAT assay after cotransfecting the respective promoter constructs with the HTLV-I Tax expression vector and with a plasmid expressing either wild-type or various mutants (143A, 248W, and 273H) of p53 into Jurkat cells. All gene expression tested was significantly inhibited by expression of wild-type p53 (Fig 6). On the other hand, all of the mutants tested showed an inability to inhibit the various gene expression. Wild-type and mutant p53s did not affect the level of basal transcription from the cellular gene promoters (data not shown).
Effect of p53 on stimulation of different cellular gene promoters by Tax. Jurkat cells were cotransfected with the promoter-CAT constructs indicated (10 μg) and 5 μg Tax expression vector (BC3.9 Sph) or the parental vector (BC12), together with expression vectors for wild-type p53, mutant p53s (143A, 248W, and 273H), or parental vector pCMV-NeoBam (5 μg). (▪) − Tax; (▨) + Tax.
Effect of p53 on stimulation of different cellular gene promoters by Tax. Jurkat cells were cotransfected with the promoter-CAT constructs indicated (10 μg) and 5 μg Tax expression vector (BC3.9 Sph) or the parental vector (BC12), together with expression vectors for wild-type p53, mutant p53s (143A, 248W, and 273H), or parental vector pCMV-NeoBam (5 μg). (▪) − Tax; (▨) + Tax.
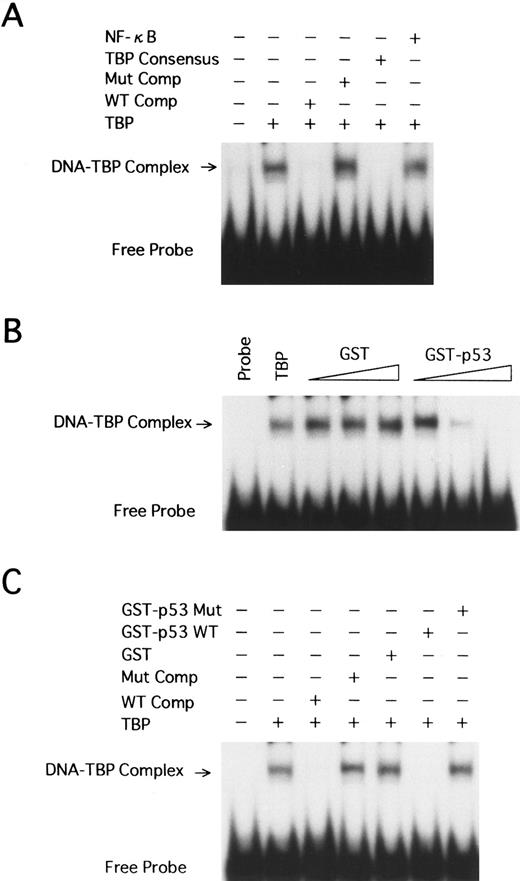
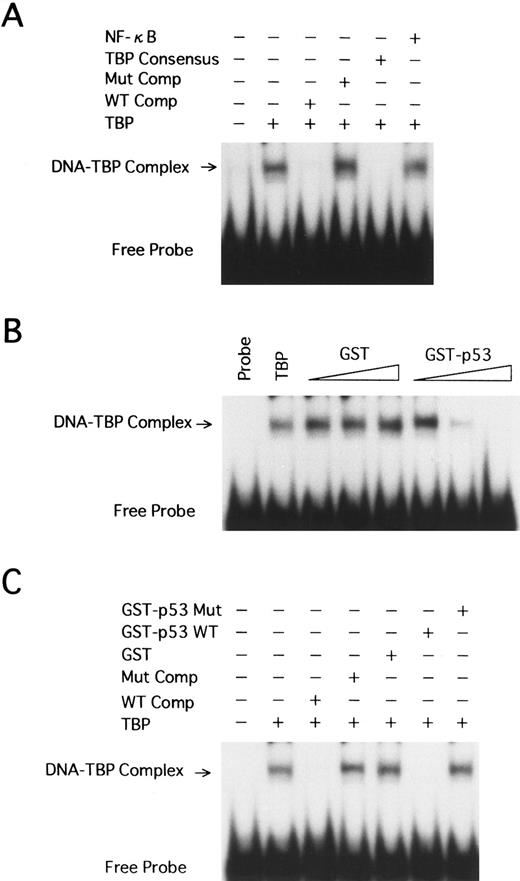
Wild-type p53 interferes with the binding of human TBP to the TATA motif of HTLV-I LTR. We have shown that wild-type p53 represses transcription from the HTLV-I promoter. This suggests that p53 may exert its effect through interaction with the TBP. TBP is essential for transcription from TATA-containing promoters. We used EMSA to study the interaction between TBP and p53 in regulation of the HTLV-I LTR. A labeled synthetic DNA probe extending from position −40 to −10 of the HTLV-I promoter, which contains the TATA box, was incubated together with purified TBP (1 FPU) at room temperature. A specific major retarded band was formed (Fig 7A, lane 2). This band could be competed away specifically by addition of a 250-fold excess of the homologous cold competitor (Fig 7A, lane 3), but not by a 250-fold excess of the identical fragment containing the mutant TATA box (Fig 7A, lane 4) and a nonspecific NF-κB oligonucleotide (Fig 7A, lane 6). A TBP consensus oligonucleotide containing a TATA box also competed with the complex (Fig 7A, lane 5).
EMSA using human TBP and HTLV-I LTR TATA region. (A) A labeled DNA fragment extending from −40 to −10 was used in binding reactions with TBP (1 FPU). Competition reactions were performed with excess amounts of the homologous (WT Comp, 250-fold) or heterologous (250-fold) competitors, including the HTLV-I LTR fragment with the TATAA-to-AGAAC 5-bp mutant TATA (Mut Comp), a TBP consensus oligonucleotide with a TATA box, and an NF-κB oligonucleotide. The TBP-TATA complex band (arrow) and unbound radiolabeled probe (Free Probe) are indicated. (B) The same probe as in A was incubated with TBP (1 FPU), GST, or GST-p53 wild-type at 3 levels: 50, 100, and 200 ng. (C) 200 ng GST, GST-p53 wild-type, or GST-p53 with a mutation at codon 135 (GST-p53 Mut) was incubated to reaction mixtures with TBP.
EMSA using human TBP and HTLV-I LTR TATA region. (A) A labeled DNA fragment extending from −40 to −10 was used in binding reactions with TBP (1 FPU). Competition reactions were performed with excess amounts of the homologous (WT Comp, 250-fold) or heterologous (250-fold) competitors, including the HTLV-I LTR fragment with the TATAA-to-AGAAC 5-bp mutant TATA (Mut Comp), a TBP consensus oligonucleotide with a TATA box, and an NF-κB oligonucleotide. The TBP-TATA complex band (arrow) and unbound radiolabeled probe (Free Probe) are indicated. (B) The same probe as in A was incubated with TBP (1 FPU), GST, or GST-p53 wild-type at 3 levels: 50, 100, and 200 ng. (C) 200 ng GST, GST-p53 wild-type, or GST-p53 with a mutation at codon 135 (GST-p53 Mut) was incubated to reaction mixtures with TBP.
Recombinant GST, GST-p53 wild-type, and codon 135 mutant GST-p53 (GST-p53 mut) were included in the EMSA to determine if p53 influences TBP binding to the TATA motif in the HTLV-I promoter. Increasing amounts (50, 100, and 200 ng) of GST-p53 fusion protein, but not GST, interfered with the TBP-TATA band formation in a dose-dependent manner (Fig 7B, lanes 3 to 8). In contrast, up to 200 ng GST-p53 mut did not show any effect (Fig 7C, lane 7).
DISCUSSION
It has been shown that p53 can either activate or suppress the activity of a number of target genes. Gene activation usually involves interaction of p53 with a specific consensus sequence, whereas it is thought that gene suppression involves interaction of p53 with the basal transcription machinery.38-40 A consensus DNA binding half site for wild-type p53 has been identified (Pu-Pu-Pu-C-A/T-T/A-G-Py-Py-Py) and shown to mediate most of the gene-stimulatory effects of wild-type p53.51 Sequence analysis of the HTLV-I LTR and cellular gene promoter regions studied here showed the absence of sites highly related to the consensus sequence. The fact that the inhibitory effect of p53 is exerted on a wide variety of promoters both viral and cellular suggests that p53 probably affects one or more of the common generalized transcription factors or that it binds to promoter sequences nonspecifically and inhibits transcription. The transient transfection results indicated that the effect of p53 on the HTLV-I LTR might be due to an interaction between p53 and Tax. As in previous reports, we failed to identify a complex between Tax and p53.21,22 Tax stimulates HTLV-I gene expression through conserved 21-bp repeat enhancer elements located in the LTR of the virus.28,52,53 We used a gel-shift analysis using the 21-bp element derived from the HTLV-I LTR to determine whether p53 inhibits complex formation. However, no significant difference in complex formation was seen between p53-transfected and p53-untransfected Jurkat cells, which transiently express Tax (data not shown), demonstrating that p53 does not seem to affect binding of the nuclear factor(s) to the 21-bp enhancer element. Wild-type p53 in cells that constitutively express Tax protein inhibited transactivation of the HTLV-I LTR by Tax (Figs 4 and 5), suggesting that p53 inhibits Tax activity after Tax protein is made. This possibility is particularly attractive, since both Tax and wild-type p53 physically interact with TBP.54 55 Therefore, TBP could mediate an antagonism of p53 against Tax. A synthesized oligonucleotide extending from position −40 to −10 bearing the TATA box was subjected to further analysis. We found that GST-p53 wild-type, but not control GST, could preclude the binding of TBP to this TATA motif in a dose-dependent manner, most probably through protein-protein interaction. In contrast, the codon 135 mutant GST-p53 had no effect. As a result, TBP is no longer able to assemble a functional transcription initiation complex.
Transfection assays have shown that wild-type p53 repressed Tax-mediated transcription from viral and cellular gene promoters. However, the levels of basal transcription from both viral and cellular promoters generated by CAT reporter plasmids tested herein were very low. Therefore, wild-type p53 did not seem to affect the level of basal transcription. Since TBP is required for Tax-activated transcription and basal transcription, wild-type p53 might affect basal transcription in the absence of Tax. We examined whether p53 represses basal transcription using the luciferase reporter plasmids. Wild-type p53 tested in the absence of Tax had little suppressive effect on the basal activity of IL-8 reporter luciferase plasmids, while p53 dramatically repressed Tax-mediated transcription from IL-8 promoter (data not shown). These findings could account for the results of gel-shift analysis performed in the absence of Tax.
We have shown that wild-type p53 is a potent endogenous inhibitor of Tax-mediated transactivation of HTLV-I LTR. What effect does endogenous p53 have on this viral promoter in the course of viral infection? Wild-type p53 might keep the virus and Tax in check in latently infected cells. Tax is originally a device for activation of viral gene expression. However, continuous expression of the viral antigens would be a target for the host immune response, and thus, these infected cells should be rejected if they continue to express Tax. p53 could exert a feedback control on HTLV-I gene expression. Endogenous p53 might play a crucial role in the survival of proviruses.
Inactivation of p53 may stimulate HTLV-I replication in individual infected cells, thus leading to accumulation of Tax. Tax protein is essential for efficient HTLV-I replication. Thus, this p53 inactivation may be important in the establishment of productive viral infection. Interestingly, Uittenbogaard et al41 described the transcriptional repression of p53 by Tax protein. These lines of evidence suggest reciprocal interactions between p53 and Tax.
We have also shown herein that overexpression of wild-type p53 can exert an inhibitory effect on Tax-mediated transactivation of a variety of cellular gene promoters. Tax alters gene expression through direct interactions with cellular transcription factors that bind to the Tax-responsive elements. These cellular factors include members of the NF-κB protein family, the activating transcription factor (ATF )/cyclic adenosine monophosphate–responsive element binding protein (CREB) family, and the serum response factor. Interaction of Tax with the ATF/CREB family of transcription factors is critical for regulation of gene expression from the viral LTR.56,57 A number of cellular genes, including those for IL-2R α chain, IL-1α, IL-6, and GM-CSF, are activated by Tax through induction of NF-κB.31-33,36 Tax can also transactivate gene expression by interacting directly with components of the basal transcriptional complex such as TBP.55 Wild-type p53 might be able to compete with Tax for binding to TBP in these promoters, resulting in inhibition of Tax-dependent transcriptional activation, since p53 inhibits Tax independently of these cellular transcription factors.
Inactivation of p53 would result in abnormal polyclonal proliferation of infected cells, because Tax protein is known to activate many cellular genes required for T-cell growth. As has been well established, leukemic cells of ATL are monoclonal. p53 inactivation could also explain such monoclonal selection of an infected cell for abnormal proliferation, since p53 inactivation could relax the G1 checkpoint and permit accumulation of chromosomal aberrations. Eventually, p53 inactivation might lead to ATL. ATL is classified into at least three stages: smoldering, chronic, and acute. p53 mutations were detected in acute ATL and not in less aggressive types of ATL (eg, chronic and smoldering),58 suggesting that p53 is involved in progression to the acute phase of ATL. Thus, inactivation of p53 is postulated to be a key event in the etiology and/or progression of ATL.
To determine whether the inhibition of Tax-mediated transactivation of HTLV-I LTR by p53 is cell type–specific, we chose also to use a monocytoid cell line (U937). The results indicate that in the U937 cell line, wild-type p53 is significantly less efficient in inhibiting Tax-mediated activation of HTLV-I as compared with the T-cell lines Jurkat and HuT78 (Figs 2 and 3). Transactivation efficiencies of PG13-CAT reporter by wild-type p53 were almost the same in all three cell lines as compared by CAT assay (Fig 1). The difference in the extent of inhibition by the same amount of wild-type p53–expressing construct indicates that cellular factors are important for promoter inhibition by p53 and that the observed promoter inhibition is possibly not an effect of general lethality caused by p53.
For the malignant growth of infected cells in ATL, genetic alterations in host cell function may be required. Our data support the notion that the functional impairment of p53 is related to the development of ATL. Further studies examining the functional activity of p53 in clinical ATL samples and in HTLV-I–transformed cell lines may lead to identification of novel mechanisms for development of leukemia. Thus, although our findings do not fully explain the mechanism by which HTLV-I leads to development of ATL, they suggest that functional inactivation of p53 represents one step in a multistep process of leukemogenesis.
ACKNOWLEDGMENT
We thank Dr B. Vogelstein for providing pC53 expression and analysis vectors.
Supported in part by National Institutes of Health Grant No. R01-DK46484.
Address reprint requests to Naoki Mori, MD, Department of Preventive Medicine and AIDS Research, Research Field of Pathogenesis and Clinical Sciences, Institute of Tropical Medicine, Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852, Japan.