Abstract
To understand the role of key molecules in determining the strength and nature of allogeneic T-cell response to leukemia, we transfected HLA-DR1 into the major histocompatibility complex (MHC)-deficient, natural killer (NK)-cell sensitive K562 leukemia cell line. Untransfected K562 cells stimulated NK proliferation in vitro and formed subcutaneous tumors in severe combined immunodeficiency/non-obese diabetic (SCID/NOD) mice. Tumor growth was inhibited by adoptive intravenous transfer of fresh unprimed peripheral blood mononuclear cells (PBMC). In contrast, HLA-DR1 transfected cells stimulated CD4+ T cells, but not NK-cell proliferation in vitro and formed tumors resistant to fresh PBMC in SCID/NOD mice. Tumors not expressing MHC were infiltrated with CD16+CD56+ lymphocytes whereas nonregressing HLA-DR1 expressing tumors showed only a scanty infiltration with both T-cell and NK-cell subsets. The results indicate that MHC class II expression by leukemia cells can determine the effector cell type that it engages. In vivo MHC class II expression rendered K562 cell tumors resistant to NK-cell mediated antitumor reactivity.
THERE IS experimental and clinical evidence that the curative effect of allogeneic marrow transplantation in hematological malignancies is mediated by donor derived immunocompetent cells.1-3 The donor alloresponse to leukemia (or graft-versus-leukemia [GVL] effect) after allogeneic bone marrow transplantation (BMT) involves both major histocompatibility complex (MHC) restricted CD4+ and CD8+ T lymphocyte and non-HLA restricted natural killer (NK) cells.4-8 The GVL effect contributes to the risk of relapse after allogeneic BMT and is responsible for the response of relapsed leukemias to treatment with donor lymphocytes after BMT. Clinical experience has shown that the potency of the GVL response varies with the type of leukemia treated. Chronic myelogenous leukemia (CML) in chronic phase is most susceptible, acute myelogenous leukemia (AML) has intermediate susceptibility, and acute lymphoblastic leukemia is the least susceptible, showing high relapse rates after allogeneic BMT and infrequent responses to treatment for relapse after BMT with donor lymphocyte transfusions.9,10 This suggests that the behavior of the malignant cell as stimulator and target of the alloresponse plays a central part in determining the strength and nature of the GVL effect. As a model to understand the role of key molecules that control the alloresponse, we transfected the MHC class I and II deficient K562 leukemia cell line with HLA-DR1. We previously showed that the susceptibility of K562 cells to NK-mediated cytotoxicity was abrogated in K562 cells expressing HLA-DR1. Our experiments indicated that NK cells received a negative signal from the MHC class II transfected cell, possibly mediated by recognition of a self peptide presented by HLA-DR1.11
To determine whether the in vitro changes produced by HLA-DR transfection of K562 cells translated into an altered susceptibility to alloreacting lymphocytes in vivo, we established K562 cell tumors in severe combined immunodeficiency/non-obese diabetic (SCID-NOD) mice and tested the ability of adoptively transferred human peripheral blood mononuclear cells (PBMC) to control tumor growth. We show here that the expression of HLA-DR1 protected K562 cells from a PBMC-mediated antitumor effect.
MATERIALS AND METHODS
Establishment of HLA-DR1 expressing K562 cell line. Details of the procedure for transfecting HLA-DRβ1*0101 gene into K562 have been described previously.11 Briefly, 10 μg of plasmid DNA for both the HLA-DRβ1*0101 α- and β-chain were coprecipitated and 5 × 106 K562 cells were transfected with the DNA by electroporation. Cells were selected in medium containing neomycin and subsequently cloned to further select by flow-sorting for cells with high surface expression of HLA-DR. Expression of HLA-DR was confirmed by specific HLA typing. K562 cells transfected only with the β-chain not expressing HLA-DR on the cell surface and untransfected K562 cells were used as controls.
Flow cytometric study of cell phenotypes. Surface phenotype of transfected K562 cells were compared with untransfected cells by flow cytometry. Monoclonal antibodies (MoAbs) specific for HLA-class I, class II, CD13 (myeloid antigen), CD15 (myeloid antigen), glycophorin-A (myeloid antigen), CD33 (myeloid antigen), CD54 (intracellular adhesion molecule-1), CD71 (transferrin receptor), CD75 (sialyl transferase), CD80 (B7, costimulatory molecule), and CD95 (fas-antigen) were used. MoAbs with specificity for T cells and NK cells (CD3, CD4, CD8, CD56, CD16) (Becton Dickinson, CA) were also used to study lymphocyte subsets. The antibodies were conjugated either with fluorescein isothiocyanate or with phycoerythrin. Cells were incubated with each of the antibodies for 30 minutes on ice and then washed 3 times in phosphate-buffered saline (PBS). The cells were analyzed using the Epics Flow cytometer (Coulter Corp, Hialeah, FL).
SCID/NOD mice and K562 tumor formation. Breeding pairs of NOD/LtSz-scid/scid mice12 were kindly provided by Leonard Shultz (Jackson Laboratory, Bar Harbor, ME). Breeding and maintenance were performed in micro isolator cages under sterile conditions. Access to food and drinking water was unrestricted. Drinking water was acidified and supplemented with Sulfatrim (200 mg Sulfamethoxazole, 40 mg Trimethoprim in 250 mL water; Barre-National Inc, Baltimore, MD) for pneumocystis carinii pneumonia prophylaxis. All experiments were performed on 4- to 8-week-old female mice with no more than a 2-week spread in a single experiment. Mice were screened for immunity before tumor inoculation by assessing murine IgG and IgM in serum obtained from retro-orbital sinus-bleed. No evidence for immune-competence was detected by the Ochterlony technique.
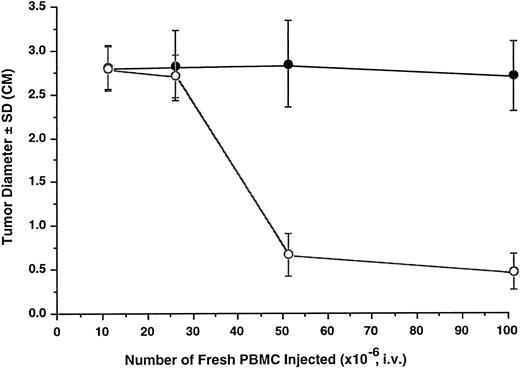
Antitumor effect of unprimed human lymphocytes in SCID mice. Tumors were initiated by inoculation of 2 × 107 K562 cells (○) or K562DR1 cells (•) through SC injection. PBMC were administered by tail vein injection 24 hours later and tumor size (maximum tumor diameter) was measured after 14 days. Each data point in the graph represents the mean and one standard deviation (SD) of 8 to 12 tumors.
Antitumor effect of unprimed human lymphocytes in SCID mice. Tumors were initiated by inoculation of 2 × 107 K562 cells (○) or K562DR1 cells (•) through SC injection. PBMC were administered by tail vein injection 24 hours later and tumor size (maximum tumor diameter) was measured after 14 days. Each data point in the graph represents the mean and one standard deviation (SD) of 8 to 12 tumors.
K562 cells were suspended in up to 0.6 mL PBS per inoculum and injected subcutaneously (SC) at varying concentrations as indicated for different experiments into the flanks of recipient mice. To determine in vivo growth of K562 cells and optimal dose for tumor formation, 3 to 5 mice were used for each data point. The effect of adoptively transferred lymphocytes on tumor formation was determined in groups of 8 to 12 mice for each treatment condition. All experimental data were reproduced at least twice.
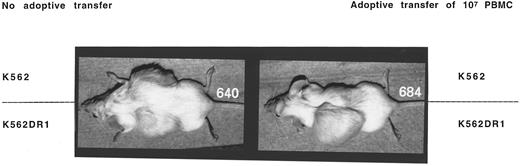
Adoptive transfer of PBMC prevented tumor formation by K562 or K562β cells, but not K562DR1 cells.
Adoptive transfer of PBMC prevented tumor formation by K562 or K562β cells, but not K562DR1 cells.
In vivo tumor formation was monitored by size measurements for up to 3 weeks post inoculation. At the termination point of the experiment, mice were killed by CO2 inhalation and tumors were subsequently excised in toto for further analysis, including mass determination, flow cytometry, and immunohistochemistry.
Preparation of effector cells and immunotherapy of mice. Effector cells were obtained by leukapheresis of five normal volunteer donors after informed consent. Their HLA DR types were DR4,16; DR 2,2 and DR 1,1. DR 7,7; (2 donors) Four DR1 negative donors were used for in vivo experiments. PBMC were separated by centrifugation of blood on Ficoll-Hypaque (Organon-Teknika, Durham, NC) density gradient. PBMC were washed 3 times in RPMI 1640 medium containing 2 mmol/L Glutamine (Life Technologies, Gaithersburg, MD), 10% fetal calf serum (Atlanta Biologicals, Norcross, GA), and antibiotics and were used fresh at doses indicated in figures. The effector cells were administered by tail vein injection 24 hours after inoculation of K562 cells.
Lymphocyte subsets were selected by negative depletion using magnetic beads conjugated with appropriate MoAbs (Dynal Ltd, Oslo, Norway). A CD4+ T-cell subset was obtained by depleting CD8+, CD56+, and CD19+ cells from PBMC; similarly a CD8+ T-cell subset was obtained by depleting CD4+, CD56+, and CD19+ cells. A NK-cell line was established as described previously11 and maintained in culture with RPMI-1640 medium supplemented with interleukin-2 (IL-2) and phytohemagglutinin.
Immunohistochemical staining. All tumor tissue was excised and stored for further study. A portion of the tumor tissue was preserved in 6% buffered formalin and embedded in paraffin. Standard techniques for immunohistochemical staining were used. Briefly, tissue sections of 5 μm were mounted onto slides and labeled with antihuman NK MoAb (NK-1; Vector Laboratories, Burlingame, CA) and developed with an alkaline Phosphatase/New-Fuchsin detection system (Kirkegaard and Perry, Gaithersburg, MD). Specimens from mice that did not receive PBMC treatment were used as negative controls.
Mixed lymphocyte-K562 cell culture. For 3H-thymidine incorporation assay, mixed lymphocyte-K562 cell cultures were set up in triplicate in 96-well round-bottomed plates at a responder to stimulator (R:S) cell ratio of 10:1. The cultures were maintained in RPMI-1640 supplemented with 10% human AB serum. After 5 days, 1 μCi of 3H-thymidine was added to each well. The cells were procured 18 hours later and 3H-thymidine incorporation was determined using a Beta Scintillation counter (Wallac, Gaithersburg, MD). For phenotypic study, the mixed cells were cultured in flasks at R:S ratio 10:1 and maintained for at least a week. The cells were then washed and stained with antibodies for flow cytometry analysis. K562 cells or K562DR1 transfectants were irradiated at 200 Gy. Background proliferation did not exceed 2 × 103 counts per minute after 6 days in culture.
Cytotoxicity assay. Details of the method have been described previously.11 Briefly, effector cells at dilutions to achieve 103 to 105 cells/well were plated in 40 μL, 60-well Terasaki trays in 6 replicates per dilution. Target cells were stained with Calcein-AM (Molecular Probes Inc, Eugene, OR). After washing 3 times in PBS, cells were resuspended and 103 cells in 10 μL medium were added to each well containing effector cells. Wells with target cells alone and medium alone were used for maximum (Mx) and minimum (Mn) fluorescence emission, respectively. After 4 hours incubation, the trays were centrifuged before measurement of fluorescence using an automated Lambda FluoroScan (One Lambda, Canoga Park, CA).
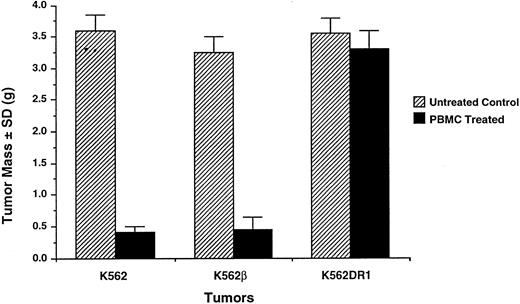
Resistance of K562DR1+ tumors to adoptively transferred PBMC. Two weeks after SC inoculation of 2 × 107 K562 or K562β or K562DR1 cells followed by intravenous administration of 5 × 107 human PBMC, all tumors were excised, trimmed of surrounding tissue, and weighed (gram). A typical experiment is shown.
Resistance of K562DR1+ tumors to adoptively transferred PBMC. Two weeks after SC inoculation of 2 × 107 K562 or K562β or K562DR1 cells followed by intravenous administration of 5 × 107 human PBMC, all tumors were excised, trimmed of surrounding tissue, and weighed (gram). A typical experiment is shown.
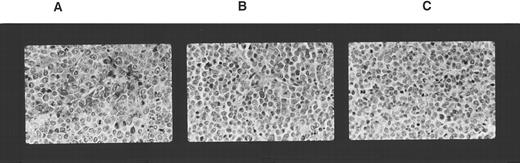
Lymphocyte subsets infiltrating tumor tissue. Immunological staining of tumor sections excised 2 weeks after adoptive transfer of PBMC. The sections were fixed in paraffin and stained with anti-NK1 MoAb. (A) K562 cell tumor; (B) K562DR1 cell tumor; (C) K562 cell tumor in the absence of adoptively transferred PBMC.
Lymphocyte subsets infiltrating tumor tissue. Immunological staining of tumor sections excised 2 weeks after adoptive transfer of PBMC. The sections were fixed in paraffin and stained with anti-NK1 MoAb. (A) K562 cell tumor; (B) K562DR1 cell tumor; (C) K562 cell tumor in the absence of adoptively transferred PBMC.
Statistics. A paired Student's t-test was used to analyze the probability of difference between cytotoxicity of effector cells against the same target.
RESULTS
Phenotype of K562 and K562DR1 transfectants. Phenotypes of transfected cells were compared with untransfected cells to detect alterations caused by the gene transfection. HLA-class II antigen was highly expressed only on K562DR1 cells, but not on K562β or K562 cells. There were no significant changes in myeloid markers CD13, 15, 33, and glycophorin-A between cells transfected with HLA-DR1 (K562DR1) or only the β-chain (K562β) and untransfected cells (K562). Similarly the nonmyeloid-specific antigens transferrin, CD54, CD80, CD95, and HLA class I did not change after DNA transduction.
K562 cell tumor formation in SCID/NOD mice. The kinetics of tumor formation was investigated to achieve an optimal K562 cell dose for reproducible tumor formation after inoculation. The SC route of injection of tumor cells was chosen to easily monitor growth by sequential measurements of the tumor diameter. Increasing doses of K562 cells were inoculated SC into the left or right flank. Initial experiments showed that tumors grew in the course of about 3 weeks to diameter >2.5 cm. Subsequent measurements were made on 14-day tumors. The tumor growth was found to be reproducible and correlated with the initial number of cells inoculated between 10 × 106 and 60 × 106 cells per flank. Furthermore, the growth kinetics of K562DR1, K562β, and K562 tumors was identical (data not shown). Repeated analysis of K562DR1 cells obtained after passage in the tumor showed no alteration in the expression of HLA-DR1 and other surface antigens.
Antitumor effect of fresh human lymphocytes in SCID/NOD mice. The tumor model was then used to investigate the antitumor effect of adoptively transferred fresh human lymphocytes from two normal donors. Mice were first inoculated with 20 × 106 K562 cells SC and then received varying doses of fresh human PBMC by tail vein injection 24 hours later. Tumor growth was determined 14 days later. The antitumor effect was detectable at an effector to target (E:T) ratio of 1:1. Fifty percent tumor inhibition was achieved at an E:T ratio of 2:1; and 80% at an E:T ratio of 5:1 (Fig 1).
HLA-DR1 expression rendered K562 cells resistant to the antitumor effect of fresh PBMC. The effect of HLA-DR1 expression by K562 cells on the antitumor effect of fresh PBMC was studied. K562DR1 transfected and untransfected K562 cells were simultaneously inoculated SC into opposite flanks. The mice received varying doses of freshly prepared PBMC by tail vein injection. Growth of K562 cell tumors was inhibited, while K562DR1 innocula formed tumors (Fig 2). Neither the tumor diameter nor the tumor mass of the K562DR1 cell was reduced by adoptive transfer of PBMC (Figs 1 and 3). Tumors of transfected and untransfected K562 cell were excised and infiltrating lymphocytes stained immunohistochemically. NK cells predominated, forming >90% of the tumor infiltrating lymphocytes in the K562 cell tumors. This proportion considerably exceeded the 10% to 15% of NK cells in the PBMC administered. In contrast there was no predominance of NK cells in the K562DR1 tumors (Fig 4).
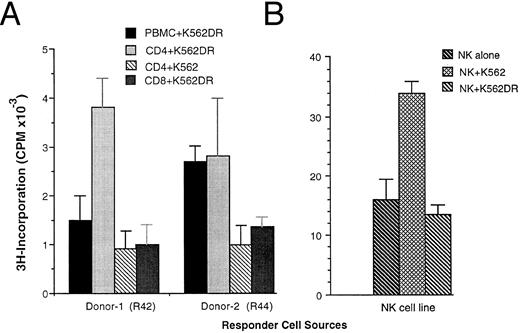
Presence of HLA-DR inhibited NK-cell activity, but induced T-cell proliferative responses. The ability of HLA-DR transfection to regulate the nature of the effector response to K562 cells was further studied in vitro. Both untransfected and transfected K562 cells induced lymphocyte proliferation as determined by 3H-Thymidine incorporation in a dose-dependent manner. However, when purified lymphocyte subsets were used as responders, the K562DR1 transfected line induced predominantly CD4+ and to a lesser extent CD8+ T-cell responses, but did not induce NK-cell proliferation. In the absence of HLA-DR expression, K562 cells induced NK-cell proliferation, but had no stimulatory effect on purified CD4+ cells (Fig 5). These changes concorded with lymphocyte phenotype analysis. T- and NK-cell lymphocyte subsets were measured by flow cytometry after 7 days in mixed K562-lymphocyte culture. Cells of the NK phenotype CD3−CD16+CD56+ decreased from 16% to 8% after 7-day culture with K562DR1, but increased from 16% to 29% after 7-day culture with K562 cells when compared to nonstimulated controls. The proportion of T cells increased after 7-day mixed culture with K562DR1 cells, but showed no significant changes when cultured with K562 cells (Table 1).
Immune responses of lymphocyte subsets to K562 and K562DR1 stimulation. Fresh PBMC or purified CD4+ or CD8+ cells (A) or cells from a NK-cell line (B) were cocultured with irradiated K562 or K562DR1 cells for 5 days. Eighteen hours after 3H-pulsing, the proliferative response was determined by 3H-incorporation. Responder cells were plated in triplicate at 5 × 104 per well with 5 × 103/well stimulator cells/well. Donor-1 was HLA-DR1+ (HLA-DR1/1, DQ5/5), donor-2 HLA-DR2+ (HLA-DR2/2, DQ6/6).
Immune responses of lymphocyte subsets to K562 and K562DR1 stimulation. Fresh PBMC or purified CD4+ or CD8+ cells (A) or cells from a NK-cell line (B) were cocultured with irradiated K562 or K562DR1 cells for 5 days. Eighteen hours after 3H-pulsing, the proliferative response was determined by 3H-incorporation. Responder cells were plated in triplicate at 5 × 104 per well with 5 × 103/well stimulator cells/well. Donor-1 was HLA-DR1+ (HLA-DR1/1, DQ5/5), donor-2 HLA-DR2+ (HLA-DR2/2, DQ6/6).
NK cells recognition of K562 targets is not HLA-DR restricted. Cytotoxicity of an HLA-DR1+ and an HLA-DR1− PBMC responders to transfected and untransfected K562 cells was compared in vitro. At all effector to target ratios tested there was no difference in cytotoxicity between effectors positive or negative for HLA-DR1. All effectors showed high cytotoxicity to K562 cells and low cytotoxicity to HLA-DR1 expressing K562 cells (Table 2).
DISCUSSION
It is clear from clinical observation that after allogeneic BMT different types of leukemia vary in their tendency to relapse and to respond to donor lymphocyte transfusion after relapse.9,10 These differences may be due to variations in the malignant potential of the leukemia, or its immunogenicity that controls the strength of the GVL alloresponse. Surface expression of a few key molecules determines the capacity of a malignant cell to stimulate an immune response and render it susceptible to immune attack. MHC molecules,13 adhesion molecules,14 and costimulatory molecules15 have all been implicated in tumor immunogenicity and probably play similar roles in the GVL response.
Because many human leukemias express MHC class II, it is of interest to determine how these molecules interact with effector cells. The expression of MHC class II on human leukemias confers on them the ability to stimulate a CD4+ T-cell response and renders them susceptible to CD4+ cell-mediated cytotoxicity.16 Furthermore, there is clinical and experimental evidence that CD4+ cells play an important role in the GVL effect in CML after allogeneic BMT.17 There is also evidence that non-MHC restricted responses of alloreacting NK cells are involved in the GVL response.6-8,18 The interaction of NK cells with MHC molecules on their targets is complex. Different NK clones appear to recognize different MHC class I subclasses.19,20 Sensitivity to NK-mediated lysis depends on a balance of positive and negative signals through killer inhibitory receptors received by the NK cell.21 22
By expressing HLA-DR1 in the MHC class I and II deficient NK-sensitive K562 cell, it was possible to study in isolation the interaction of NK cells and T cells with a single MHC class II molecule. HLA-DR1 was chosen because it is a relatively common class II antigen in the population and the gene construct was readily available.23 We confirmed that HLA-DR1 gene transduction did not alter cell growth in vitro or tumor formation in the SCID/NOD mouse nor the phenotype of its common cell surface antigens. Because the K562β transfected cells (without surface-expressed MHC class II) did not show any differences from the untransfected cells, the effect of the K562 DR transfectant on NK-cell and T-cell activity appeared to be due only to the expressed HLA-DR1 antigen.
In this study, we showed that HLA-DR1 expression rendered the classically NK-sensitive K562 cell line resistant to NK cell-mediated lysis and inhibited NK cell proliferation while inducing a predominant CD4+ T-cell response. The in vitro resistance to NK lysis by the K562DR1 line was manifest in the SCID/NOD mouse as resistance to the antitumor effect of adoptively transferred PBMC. The antitumor effect was most likely due to NK cells because it was conferred by unprimed PBMC and CD16+, CD56+ cells predominated in the tumor.
We have previously shown that the inhibitory effect of the K562DR1 transfectant on NK lysis appeared to be mediated by endogenous peptides presented by the HLA-DR1 molecule: protection from lysis occurred equally with NK cells matched or mismatched with HLA-DR1 and was abrogated by brefeldin (an inhibitor of endogenous antigen processing).11 It is possible that the same peptides stimulated the proliferative CD4+ response since both HLA-DR1 matched and mismatched T cells responded equally to the transfected cell line. Thus, the presentation of antigen through HLA-DR1 appeared to be the central factor governing tumor susceptibility to NK cells and the alteration in effector cell response.
The accurate prediction by the in vitro studies of the antitumor effect found in the SCID mouse provides useful support for the relevance of other in vitro observations of antitumor immunity. However, in addition, our in vivo experiments showed that NK cells administered intravenously can home to the site of an experimental tumor and preferentially accumulate in an NK-sensitive tumor. This model will allow studies of the effect of mixing sensitive and nonsensitive targets in the same tumor and the effect of NK-stimulating cytokines IL-2 and IL-12 on effector function in the resistant tumor. Furthermore the model could be adapted to study the antitumor effect of T cells primed in vivo to the tumor.24
The development of in vivo models of allogeneic antileukemia responses may shed light on some of the mechanisms of GVL in clinical BMT. The variation of the strength of GVL in different types of leukemias and different stage of the same disease is not well understood. Many early leukemia progenitor cells express both MHC class I and II molecules and therefore represent good targets for T-cell attack if appropriate alloantigens were presented.25 In solid tumors, the downregulation in surface expression of MHC class I and II is a frequent occurrence which may render the tumor resistant to a T-cell attack. Such MHC deficient cells should however become targets for an NK-cell attack.22 Similar changes can occur in leukemia relapsing after BMT.26 Since many myeloid leukemias are MHC class II+27 it is interesting to speculate whether differences in class II expression might explain different susceptibilities of leukemia to the GVL effect. During myeloid differentiation CD33+ cells downregulate HLA class II. Such cells should therefore be more susceptible to NK-cell mediated killing. The stronger GVL reaction seen in CML when compared with AML9,10 could be related to the preponderance of DR negative CD33+ NK-sensitive cells in CML in chronic phase. However other key surface molecules such as B7.1 costimulatory molecules15 and fas antigen28 may have equal or greater significance in determining immunogenicity and susceptibility to killing of leukemia cells. The relative importance of these molecules in determining alloimmune responses to leukemia could be studied further using the approaches described here. In addition, the model could be used to study the way in which MHC-restricted and -nonrestricted effector cells interact to exert a GVL effect.
Address reprint requests to A. John Barrett, MD, Bldg 10, Room 7C103, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892.