Abstract
Flavopiridol is a novel, potent inhibitor of cyclin-dependent kinases (CDK). This synthetic flavone has been reported to exhibit antitumor activity in murine and human tumor cell lines in vitro and in vivo and is currently undergoing clinical phase I evaluation. In the present study, 1 Epstein-Barr virus (EBV)-transformed B-prolymphocytic cell line (JVM-2), 1 EBV-transformed B-CLL cell line (I83CLL), and 1 non-EBV transformed B-CLL cell line (WSU-CLL) were used as targets. Treatment of the cells with flavopiridol (100 nmol/L to 400 nmol/L) led to a marked dose- and time-dependent inhibition of cell growth and survival as determined using trypan blue exclusion. Morphologic analysis showed characteristic apoptotic changes such as chromatin condensation and fragmentation, membrane blebbing, and formation of apoptotic bodies. Furthermore, quantitative assessment of apoptosis-associated DNA strand breaks by in situ TdT labeling showed that a significant number of flavopiridol-treated cells underwent apoptosis. These cellular effects were associated with a significant decrease in bcl-2 expression as observed by Northern and Western blotting. The results showed that flavopiridol downregulates bcl-2 mRNA and bcl-2 protein expression within 24 hours. Genistein and quercetin, two flavonoids that do not inhibit CDKs, did not affect bcl-2 expression. These data suggest an additional mechanism of action of this new flavone which might be useful as an agent in the treatment of chronic lymphoid malignancies.
FLAVONOIDS SUCH as genistein or quercetin have been shown to inhibit tumor cell growth.1 Flavopiridol is a new synthetic flavone that has been demonstrated to have growth inhibitory activity in vitro and in vivo in numerous human tumor cell lines and xenograft models.2-4 The agent has been found to induce a blockade of cell-cycle progression at G1 or G2 interfaces, in association with direct inhibition of CDK2, CDK4, and CDK1.5,6 Interestingly, flavopiridol has been shown to enhance the cytotoxic effect of conventional chemotherapeutic agents in gastric and breast cancer cell lines.7 A phase I clinical trial has recently been performed to evaluate the potential antineoplastic effect of this compound.8
Chronic lymphocytic leukemia (CLL), the most frequent type of adult leukemia in Western countries, is a malignant disorder characterized by the progressive accumulation of clonally derived, CD5+ , CD19+ B lymphocytes.9 It has been suggested that prolonged life span, due to dysregulation of apoptosis, is a major factor contributing to the delayed senescence of these transformed lymphocytes.10 In this regard, the proto-oncogene bcl-2, which delays apoptosis, has been documented to be highly expressed in most B-CLL,11 although a translocation of the gene occurs infrequently in this disease.12 Overexpression of bcl-2 has been associated with resistance to chemotherapy and inhibition of apoptosis.13 14
In this study we investigated the ability of flavopiridol to induce apoptosis in chronic lymphoid malignancies of the B-cell type including CLL and prolymphocytic leukemia (PLL). In addition, we show for the first time that the growth inhibition and induction of apoptosis observed in CLL cells exposed to flavopiridol is associated with the ability of this agent to downregulate the expression of bcl-2 mRNA and protein.
MATERIALS AND METHODS
Reagents. Flavopiridol (L 86-8275, [(−) cis-5,7-dihydroxy-2-(2-chlorophenyl ) - 8 - ;ob 4 - ( 3 - hydroxy - 1 - methyl ) - piperidinyl ;cb - 4H - 1 - ben -zopyran-4-one]) was dissolved in sterile H2O at a stock concentration of 100 μmol/L and stored at −20°C. Serial dilutions (200 to 800 nmol/L) were made in medium. The agent was kindly provided by Dr Edward A. Sausville (National Cancer Institute, NIH, Bethesda, MD). Genistein and quercetin (both from Calbiochem, La Jolla, CA) were dissolved in dimethyl sulfoxide (DMSO) at stock solutions of 10 mmol/L. Further dilutions (10 to 50 μmol/L) were set up in media. Fludarabine des-phosphate (FAra, 9-β-D-arabinosyl-2-fluoroadenine) (Sigma, St Louis, OH) was dissolved in 100% DMSO for a stock solution of 10 mmol/L. It was further diluted in medium at final concentrations of 1 to 5 μmol/L.
Cell lines. The well-characterized permanent human B-cell leukemia cell line JVM-215 was originally established from the peripheral blood of a patient with B-prolymphocytic leukemia (PLL) by Epstein-Barr virus (EBV)-immortalization. The human chronic B-cell leukemia cell line I83CLL was derived from the peripheral blood of a patient with B-cell chronic lymphocytic leukemia (B-CLL) by EBV-immortalization.16 The human EBV− chronic B-cell leukemia cell line WSU-CLL was established from a patient with advanced B-CLL17 and the human promyelocytic cell line HL-60 (used as a positive control for bcl-2 expression) were cultured in RPMI 1640, supplemented with 1% penicillin/streptomycin, 1 mmol/L L-glutamine (GIBCO-BRL, Grand Island, NY), 10% heat-inactivated fetal bovine serum (FBS) (GIBCO-BRL) at 37°C, 5% CO2 in air.
Viability and cell growth. Loss of cell viability and cell growth as measured by trypan blue (GIBCO-BRL) exclusion were assessed at specified time points in suspension cultures. At selected time points, samples, performed in triplicate, were collected and evaluated.
Nonradioactive Northern blot analysis. Total cellular RNA (10 μg/lane) was size-fractionated by electrophoresis in a 1.2% agarose gel containing 0.7 mol/L formaldehyde, transferred to nylon membranes (Hybond N; Amersham, Arlington Heights, IL) by capillary suction overnight, and crosslinked by UV-light (Stratalinker; Stratagene, La Jolla, CA). The cDNA probe for bcl-2 (generously provided by Dr Andrew Zelenetz, Department of Medicine, Lymphoma Service, Memorial Sloan-Kettering Cancer Center, New York, NY), a 410-bp EcoRI fragment in pCR II vector (TA cloning vector; Invitrogen, San Diego, CA), digested with BamHI, was labeled with digoxygenin-UTP (DIG-UTP) (Boehringer Mannheim, Indianapolis, IN) by in vitro transcription with respect to T7 RNA-polymerase. The cDNA probe for glyceraldehyde-phosphate-dehydrogenase (GAPDH), a 1,270-bp insert in pBS KS+ (American Type Culture Collection [ATCC], Rockville, MD), was linearized with EcoRI and labeled with DIG-UTP by in vitro transcription with respect to T7 RNA-polymerase. Hybridization was performed as previously described.18 Detection was accomplished by chemiluminescent reaction according to the Genius System (Boehringer Mannheim) as previously described.18
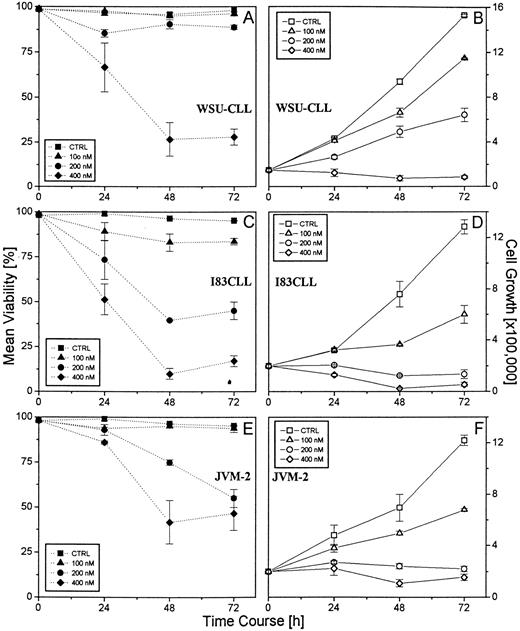
Effect of flavopiridol on the viability and cell growth of the B-CLL cell lines WSU-CLL, 183CLL, and JVM-2. 1.5 × 105 cells/mL were incubated in the presence of various concentrations of flavopiridol (100, 200, 400 nmol/L) over a time period of 72 hours. Untreated cells were used as control. Mean viability was assessed using trypan blue dye exclusion. A representative experiment (performed in triplicate) is shown.
Effect of flavopiridol on the viability and cell growth of the B-CLL cell lines WSU-CLL, 183CLL, and JVM-2. 1.5 × 105 cells/mL were incubated in the presence of various concentrations of flavopiridol (100, 200, 400 nmol/L) over a time period of 72 hours. Untreated cells were used as control. Mean viability was assessed using trypan blue dye exclusion. A representative experiment (performed in triplicate) is shown.
In situ TdT assay. The in situ terminal deoxynucleotidyl transferase (TdT) assay was performed as previously described.19 Briefly, aliquots of cells were collected from liquid culture centrifuged, and fixed in 0.3% buffered formaldehyde (pH 7.5). After washing with phosphate-buffered saline (PBS), cells were resuspended in 70% ethanol (−20°C) and stored at −20°C. Following rehydration with PBS, the cells were resuspended in 50 μL cacodylate buffer containing 0.2 mol/L potassium cacodylate, 2.5 mmol/L Tris-HCl (pH 6.6), 2.5 mmol/L cobalt chloride (CoCl2 ), 0.25 mg/mL bovine serum albumin, 7.5 U of terminal deoxynucleotidyl transferase (TdT), and 0.5 nmol/L biotinylated deoxyuracil-triphosphphate (b-dUTP) (all reagents from Boehringer Mannheim). After an incubation period in this solution for 30 minutes at 37°C, cells were washed with PBS and resuspended in 100 μL of a solution containing 4 × salt saturated citrate (SSC), 25 μg/mL avidin-fluorescein-isothiocyanate (avidin-FITC; Boehringer Mannheim), 0.1% Triton X-100, and 5% wt/vol nonfat dry milk, and incubated for 30 minutes in the dark at room temperature. Cells were washed with PBS containing 0.1% Triton X-100 and resuspended in 0.5 mL PBS containing 5 μg/mL propidium iodide (Sigma) and 0.1% RNAse A (Sigma). Green fluorescence detecting FITC levels and red fluorescence measuring propidium iodide content of individual cells were quantified on a FACStar (Becton Dickinson, Franklin Lakes, NJ), and data from 2 × 104 cells were collected and used for analysis.
Western blot analysis. Cells were lysed in a lysis buffer containing 20 mmol/L Tris HCl, 1 mmol/L EGTA, 50 μmol/L NaVO4 , 50 mmol/L NaF, 0.01 U/mL aprotinin, 10 μg/mL leupeptin, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) (all from Sigma). The lysates were then sonicated using a ultrasonic homogenizer (4710 series; Cole Parmer Instruments, Chicago, IL), centrifuged at 7,500g in a microfuge (Sorvall Instruments, Wilmington, DE), and the protein content of the lysates was determined (BioRad Protein Assay Kit I, Melville, NY) at 595 nm with a bovine serum albumin (BSA) standard. Sample buffer containing 10% glycerol, 0.4% sodium dodecyl sulfate (SDS), 0.3% bromphenol blue, 0.2% pyronin Y, in 1 × stacking buffer (Tris base 0.5 mol/L, 0.8% SDS), 20% 2-mercaptoethanol, was added to the cell lysates that subsequently were heat-denaturated at 95°C for 3 minutes. 10 μg/lane of protein was loaded on a SDS-polyacrylamide gel containing 12.5% polyacrylamide, and size-fractionated by electrophoresis. Proteins were electroblotted onto Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA) and immunostained with a mouse-antihuman monoclonal bcl-2 antibody (1:5,000; Dako, Carpinteria, CA). Bound antibody was detected using the ECL chemiluminescence detection system (Amersham, Arlington Heights, IL). Protein bands were quantified by computer densitometry.
Detection of apoptosis-associated DNA strand-breaks by in situ terminal deoxynucleotidyl transferase assay (TdT-assay) in WSU-CLL and I83CLL cells. The cells were either untreated (CTRL) or exposed flavopiridol (200 nmol/L, 400 nmol/L) for 24 hours, respectively. The dots represent the distribution of individual cells with respect to their b-dUTP incorporation and DNA content. The degree of b-dUTP complexes correlated with DNA strand-breaks per cell.
Detection of apoptosis-associated DNA strand-breaks by in situ terminal deoxynucleotidyl transferase assay (TdT-assay) in WSU-CLL and I83CLL cells. The cells were either untreated (CTRL) or exposed flavopiridol (200 nmol/L, 400 nmol/L) for 24 hours, respectively. The dots represent the distribution of individual cells with respect to their b-dUTP incorporation and DNA content. The degree of b-dUTP complexes correlated with DNA strand-breaks per cell.
RESULTS
Flavopiridol decreases viability and arrests cell growth in CLL cell lines. JVM-2, I83CLL, and WSU-CLL cells were used as targets to investigate the effect of flavopiridol on the growth and survival. A significant decrease in cell viability was observed over time in all of the cells exposed to varying concentrations of flavopiridol. A difference in the kinetics of the response to flavopiridol varied among the three cell lines. In this regard, a 50% reduction in cell viability following exposure to 400 nmol/L flavopiridol was observed after 24 hours (I83CLL), 30 hours (WSU-CLL), and 40 hours (JVM-2), respectively (Fig 1A, C, and E).
Exposure of JVM-2, I83CLL, and WSU-CLL cells to flavopiridol resulted in a significant dose-dependent inhibition of cell growth. A dose of 400 nmol/L was required in WSU-CLL cells to induce growth arrest, whereas a concentration as low as 200 nmol/L led to comparable effects in JVM-2 and I83CLL cells.
Induction of apoptosis by flavopiridol. Morphologic analysis showed characteristic apoptotic changes such as chromatin condensation and fragmentation, membrane blebbing, and formation of apoptotic bodies in all three cell line targets after treatment with flavopiridol (200 to 400 nmol/L) (data not shown).
I83CLL and WSU-CLL cells were treated with flavopiridol and examined for apoptosis-associated DNA strand breaks using the in situ terminal deoxynucleotidyl transferase assay (TdT-assay). The intensity of labeling of apoptotic cells with biotinylated dUTP correlated with the number of DNA strand breaks per cell. The data showed that a significant number of cells exposed to flavopiridol underwent apoptosis. Treatment of WSU-CLL cells with flavopiridol (200 and 400 nmol/L) for 24 hours resulted in 9.59% and 48.67%, respectively, to be apoptotic versus control cells with 0.46% apoptosis. A similar dose-dependent response could be observed in I83CLL cells with 21% (200 nmol/L) and 53.9% (400 nmol/L) apoptotic cells as compared with 2.1% apoptosis in control cells observed at 24 hours after exposure (Fig 2).
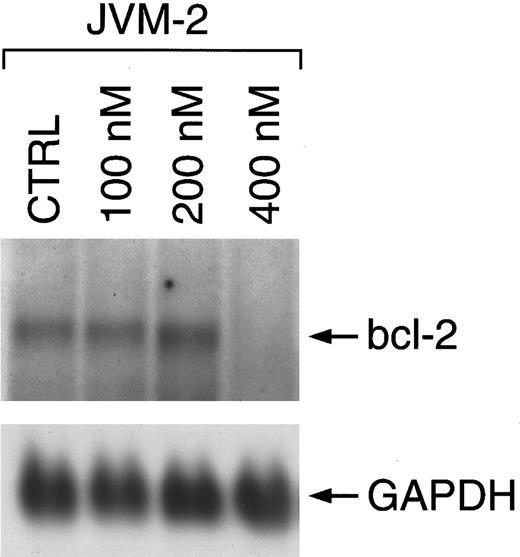
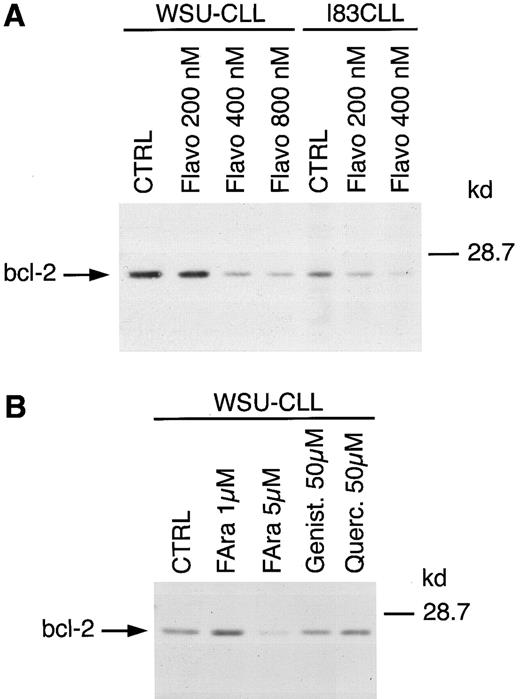
Flavopiridol decreases the expression of bcl-2. To investigate the potential effect of flavopiridol on bcl-2, we examined the expression on both mRNA and protein after a 24-hour exposure to this agent. Northern (data shown for JVM-2 cells in Fig 3) and Western blot analysis (data shown for WSU-CLL and I83CLL cells in Fig 4A) demonstrated a concentration-dependent decrease of bcl-2 expression in all three cell lines. The sensitivity to flavopiridol paralleled that observed for cell growth and loss of viability among the cell lines: A concentration of 200 and 400 nmol/L was observed to be potent in I83CLL cells whereas similar effects in WSU-CLL cells were obtained with 400 or 800 nmol/L (Fig 4A). To further evaluate whether other flavonoids such as genistein or quercetin also affect the expression of bcl-2 protein, WSU-CLL cells were exposed to these agents (10 and 50 μmol/L) for 24 hours, respectively. Western blot analysis showed no changes in bcl-2 levels following treatment with either genistein or quercetin at 50 μmol/L (Fig 4B).
Regulation of bcl-2 mRNA expression in JVM-2 cells upon treatment with flavopiridol over a time course of 24 hours. Total cellular RNA (10 μg/lane) of untreated cells (CTRL) and cells cultured in the presence of flavopiridol was hybridized using a digoxigenin-labeled bcl-2 probe. Bcl-2–specific transcripts are shown at 8.5 kb as indicated by the arrow. The lower panel shows rehybridization with a digoxigenin-labeled GAPDH probe.
Regulation of bcl-2 mRNA expression in JVM-2 cells upon treatment with flavopiridol over a time course of 24 hours. Total cellular RNA (10 μg/lane) of untreated cells (CTRL) and cells cultured in the presence of flavopiridol was hybridized using a digoxigenin-labeled bcl-2 probe. Bcl-2–specific transcripts are shown at 8.5 kb as indicated by the arrow. The lower panel shows rehybridization with a digoxigenin-labeled GAPDH probe.
(A) Regulation of bcl-2 protein expression in WSU-CLL and I83CLL cells upon treatment with various concentrations of flavopiridol. The cells (1 × 106/mL) were cultured alone (CTRL) or in the presence of flavopiridol (flavo) (200, 400, 800 nmol/L). (B) Exposure of WSU-CLL cells to fludarabine (FAra) (1, 5 μmol/L), genistein (Genist) (50 μmol/L), or quercetin (Querc) (50 μmol/L). Twenty micrograms of protein/lane from cell lysates were loaded on a 12.5% SDS-PAGE gel and electrophoresed. Bcl-2–specific protein was detected using an anti–bcl-2 monoclonal antibody (Dako).
(A) Regulation of bcl-2 protein expression in WSU-CLL and I83CLL cells upon treatment with various concentrations of flavopiridol. The cells (1 × 106/mL) were cultured alone (CTRL) or in the presence of flavopiridol (flavo) (200, 400, 800 nmol/L). (B) Exposure of WSU-CLL cells to fludarabine (FAra) (1, 5 μmol/L), genistein (Genist) (50 μmol/L), or quercetin (Querc) (50 μmol/L). Twenty micrograms of protein/lane from cell lysates were loaded on a 12.5% SDS-PAGE gel and electrophoresed. Bcl-2–specific protein was detected using an anti–bcl-2 monoclonal antibody (Dako).
We also exposed WSU-CLL cells to the purine analogue fludarabine, a conventional therapeutic agent in the treatment of CLL, which has been shown to downregulate bcl-2 in freshly isolated cells from patients with CLL.20 Our results obtained with CLL cell lines revealed no effect with fludarabine at 1 μmol/L, whereas a diminished expression of bcl-2 protein was observed at 5 μmol/L after a 24-hour exposure (Fig 4B).
DISCUSSION
In the present study we show for the first time that flavopiridol-mediated growth inhibition and induction of apoptosis is associated with a dramatic decline in bcl-2 expression in cell lines representative of B-cell chronic lymphoid malignancies.
Flavopiridol is a recently developed flavone that has been described to be more potent than its structural analogues genistein or quercetin.2 The agent reversibly inhibits tumor cell proliferation2 and induces cell death in noncycling tumor cells such as A549 human lung carcinoma cells.4 Several pathways have been suggested to be activated following administration of flavopiridol. Originally, the drug was reported to inhibit the kinase activity of CDK1 (cdc2) in breast carcinoma cell lines, which is necessary for the cell-cycle progression from G2 to M.3 More recent studies showed that flavopiridol induces G1 arrest with inhibition of CDK2, CDK4, or CDK7.5 21
Bcl-2 has been described as a repressor of apoptosis in many different cell types.22 In most B-CLL cells high levels of both bcl-2 mRNA and protein are documented; however, enhanced expression occurs predominantly in the absence of a t(14; 18) translocation of the gene.12
We report here that flavopiridol, known as an inhibitor of cyclin-dependent kinases, induces a decrease in bcl-2 expression in CLL cell lines. This suggests that flavopiridol-induced cell death involves additional mechanisms of action other than inhibition of cyclin-dependent kinases. Whether the downregulation of bcl-2 is a direct or indirect effect is not known so far. This is presently an area of investigation in our laboratory. Additional experiments with two other flavonoids, genistein23 and quercetin,24 which do not inhibit cyclin-dependent kinases, did not show a significant effect on bcl-2 expression under our experimental conditions at 50 μmol/L. Unexpectedly, at lower concentrations, genistein and quercetin (10 μmol/L) augmented intracellular levels of bcl-2 protein in this system over the same time period (data not shown). In this regard, it is of interest that Liu et al25 previously showed that genistein inhibited the ability of taxol to induce a decline in bcl-2 in a human ovarian tumor cell line system. The mechanisms by which genistein and quercetin augment bcl-2 at lower concentrations is presently under investigation in our laboratory.
Other selective CDK inhibitors such as butyrolactone I26 or roscovitine27 28 have not been reported to have an influence on the expression of bcl-2. It will be important to test whether these specific CDK inhibitors also promote downregulation of bcl-2 to determine if CDK inhibition is a crucial target for this effect. These experiments are presently underway.
In the present study, flavopiridol exhibited significant activity alone in our cell lines. Previous studies in solid tumors have failed to demonstrate significant activity of flavopiridol alone7: however, our recent data suggest that response to single-agent flavopiridol may require a decrease in bcl-2 expression in responding targets, suggesting that downregulation of bcl-2 is an important effect of this drug.29 It is of note that the purine analogue fludarabine, an agent with significant activity in CLL,30,31 has also been shown to downregulate bcl-2 mRNA in freshly isolated cells from CLL patients,20 and in our system, to promote a decline in bcl-2 protein expression in CLL cell lines following a 24-hour exposure to 5 μmol/L.
Dysregulation of apoptosis appears to be involved in the pathogenesis of CLL.32,33 Three purine analogues, fludarabine, pentostatin, and cladribine, potent inducers of apoptosis, are currently of therapeutic benefit in the treatment of CLL.34 35 In addition, the degree of apoptotic cell death induced correlates with disease status. Despite therapy, the relapse rate underscores the need to develop novel strategies for the treatment of this disease. Our data show that flavopiridol exhibits potent activity in these model systems, suggesting that it may be a useful agent in the treatment of chronic lymphoid malignancies.
Supported by grants from the Deutsche Forschungsgemeinschaft (Ko 1583/1-1) and the National Institute of Health (CA67823-01).
Address reprint requests to Andrea König, PhD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, PO Box 320, New York, NY 10021.