Abstract
CD44 molecule is a cell surface glycoprotein involved in many cell-cell and cell-matrix interactions. Circulating serum CD44 (s-CD44) levels have been found to change in parallel with response to therapy, but little is known about the predictive or prognostic significance of s-CD44. In the present study, we measured s-CD44 levels in sera taken before treatment from 194 patients with non-Hodgkin's lymphoma using a chemiluminescence-enzyme immunoassay method. All except 1 patient were regularly followed-up after therapy at least for 60 months (range, 33 to 143 months). The median pretreatment s-CD44 level was 440 ng/mL (range, 13 to 1,220 ng/mL). Only 32% of the 92 patients with an International Prognostic Index (IPI) score of 0 or 1 had an s-CD44 concentration higher than the median as compared with 67% of the patients with an IPI score ≥2 (P < .0001). Patients with lower than the median s-CD44 achieved more often a complete remission to therapy (P = .0002) and had better survival (P = .007) than those with higher s-CD44 levels. However, in a multivariate analysis, only the IPI score had independent prognostic value (P < .001). The findings were similar if only the patients with diffuse large-cell lymphoma (n = 51) were included in the analysis, but among patients with low-grade lymphoma, the median s-CD44 level was not significantly associated with the IPI or survival. In conclusion, a high s-CD44 level at diagnosis is associated with a high IPI score, poor response to treatment, and unfavorable outcome in non-Hodgkin's lymphoma.
ABOUT ONE HALF OF THE patients with non-Hodgkin's lymphoma (NHL) still die of their disease, although the cure rates have improved during the last decades. There is great clinical interest to identify prospectively patients with different survival expectations and those patients who are likely to achieve a complete response to therapy. In 1993, the International Non-Hodgkin's Lymphoma Prognostic Factors Project developed a prognostic factor model for patients with aggressive NHL.1 This International Prognostic Index (IPI), based on five different prognostic characteristics (age, performance status, number of extranodal sites, Ann Arbor stage, and serum lactate dehydrogenase concentration [s-LDH]) divides patients into four different risk groups. The IPI has been shown to be useful in all grades of lymphoma,2 3 but there is still a need to identify additional factors that predict response to therapy.
CD44 is a multifunctional adhesion molecule that is widely distributed in different cell types and tissues. Most hematopoietic cells express the standard 90-kD form, but larger forms up to 250 kD have been described.4,5 The modifications of CD44 arise from alternative splicing and/or postranslational modifications.5-7 CD44 is involved in different cell-cell and cell-matrix interactions. It has been shown to be involved in lymphocyte adhesion to vascular endothelium8,9 and the extracellular matrix,10 in T-cell activation and adherence,11 and in lymphohematopoiesis.12 Moreover, it has been described to work as a signaling molecule,13 and exon v3-containing forms have been shown to be able to bind growth factors.14 CD44 has been suggested to be involved in the metastatic process of both human malignancies and experimental animal tumors.15-18 In patients with NHL, CD44 and CD44v6 expression have been shown to correlate with survival.19-21 CD44− lymphomas appear to be more often local and have a better prognosis than lymphomas that express CD44.19 Most likely, CD44 is involved in hematogenic dissemination of NHL.
In a previous study, we found that a decrease in the serum CD44 (s-CD44) levels paralleled the attainment of a complete response to chemotherapy in patients with lymphoma.22 Elevated s-CD44 concentrations were detected at the time of diagnosis and at progression of the disease as compared with healthy controls. Similarly, in patients with gastric or colon cancer, s-CD44 measured at the time of diagnosis correlates with the tumor burden and decreases after resection of the tumor.23 Soluble CD44 has been suggested to be shed at least partly from tumor cells.24 Shedding is most likely a consequence of the activity of endogenous proteolytic mechanisms.25 Although s-CD44 lacks the cytoplasmic tail, it seems to be biologically active according to several functional studies.24 26 Based on the previous studies, s-CD44 may have importance as a marker of treatment response in patients with NHL.
There are no data available on the predictive or prognostic significance of soluble circulating CD44 in lymphoma. In the present study, we investigated the prognostic and clinical value of s-CD44 in patients with NHL and in the subgroup of diffuse large-cell lymphoma (DLCL). To this end, we developed a new method, chemiluminescence-enzyme immunoassay (EIA), suitable for measuring s-CD44 concentrations in large series of serum samples.
MATERIALS AND METHODS
Patients. s-CD44 was measured in 194 patients with NHL diagnosed and treated in the Department of Oncology, Helsinki University Central Hospital (Helsinki, Finland) from 1981 to 1987. Forty-eight patients (25%) had low-grade, 90 (46%) had intermediate-grade, and 48 (25%) had high-grade lymphoma according to the Working Formulation scheme,27 and 8 (4%) cases were considered unclassifiable. Eighty-eight (45%) of the patients were men and the median age was 59 years (range, 16 to 94 years). Clinical staging was performed according to the Ann Arbor classification system.28 The clinical status, chest x-ray, computed tomography scans of the mediastinum and abdomen, and a bone marrow biopsy were performed as staging examinations. Sixty-five (34%) of the patients had stage I, 50 (26%) had stage II, 32 (17%) had stage III, and 45 (23%) had stage IV disease at diagnosis, and in 2 cases the staging examinations had not been adequately performed. Thirty-eight (20%) had B symptoms (weight loss, unexplained fever, or night sweats). s-LDH was measured at the time of diagnosis in 184 patients, with a median value of 377 U/L (range, 170 to 3,760 U/L). The upper limit of the normal reference range for s-LDH value is 450 U/L. The IPI was determined in 183 patients.
One hundred thirty-two patients were treated with combination chemotherapy. The patients with intermediate- or high-grade lymphoma and disseminated disease were treated usually with bleo-CHOP (bleomycin, cyclophosphamide, doxorubicin, vincristine, prednisone), M-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone), or another anthracycline-containing combination chemotherapy regimen. Low-grade lymphomas were usually treated with a single-agent chlorambucil if symptomatic. In addition, 71 patients received megavoltage radiotherapy, and 37 patients were treated only with radiotherapy. Forty (56%) of the 71 patients who received radiotherapy had stage I disease. A complete response to the primary therapy was achieved in 127 cases, a partial response was achieved in 37 cases, no change was observed in 7 cases, and progressive disease was observed in 2 cases. The rest of the patients (n = 21) were considered nonevaluable, including 4 patients who died during therapy.
All patients were regularly followed-up with intervals of a few months in an outpatient department. The median follow-up time was 86 months (range, 33 to 143 months), and all except 1 patient were followed-up for more than 60 months. During the follow-up time, 99 patients died. The venous blood samples had been collected immediately before starting lymphoma therapy, and sera were stored at −20°C for 9 to 15 years before analysis.
Monoclonal antibodies (MoAbs). The MoAb Hermes-3 (mouse IgG2a) recognizes an epitope in the extracellular region of the constant part of the CD44.4 MoAb 1F1 recognizes another epitope in the constant part of the CD44 molecule. 1F1 was produced by immunization of BALB/c mice with affinity-purified CD44 antigen. Specific pathogen free BALB/c mice were immunized with affinity-purified CD44 antigen in incomplete Freund's adjuvant into the footpads three times at 1-week intervals. Lymphocytes from popliteal lymph nodes were isolated and fused with NS-1 myeloma cells using a standard procedure. Hybridoma supernatants were tested using Namalwa cells transfected either with a construct encoding the standard, hematopoietic form of CD44 or with the vector only (pcDNA neo 3) by single-color indirect immunofluorescence staining. Tonsil sections were also stained by immunohistochemistry. A detailed description of the preparation of CD44 transfectants is presented elsewhere.29 A positive hybridoma, 1F1, was subcloned twice. The isotype (mouse IgG2b) of MoAb 1F1 was determined using a mouse MoAb isotyping kit (Sigma Immunochemicals, St Louis, MO). MoAb 3G6, a mouse antibody against chicken T cells, was used as a negative control. All MoAbs were used as serum-free culture supernatants or (NH4 )2SO4 precipitated concentrates.
Immunofluorescence staining. CD44 transfectants and mock controls were incubated with hybridoma supernatants for 20 minutes, followed by the second-stage fluorescein isothiocyanate-conjugated sheep antimouse Ig (Sigma). Analyses were performed using a FACScan cytometer (Becton Dickinson, Mountain View, CA). MoAb Hermes-3 was used as a positive control and 3G6 was used as a negative control.
Epitope mapping analyses. CD44 transfectants were incubated for 20 minutes with MoAbs Hermes-3 or 1F1. After washing, the cells were incubated with biotinylated MoAbs Hermes-3 or 1F1, followed by a second-stage reagent Streptavidin-phycoerythrin (SA-PE; Becton Dickinson Immunocytometry Systems, San Jose, CA). Analyses were performed using a FACScan cytometer.
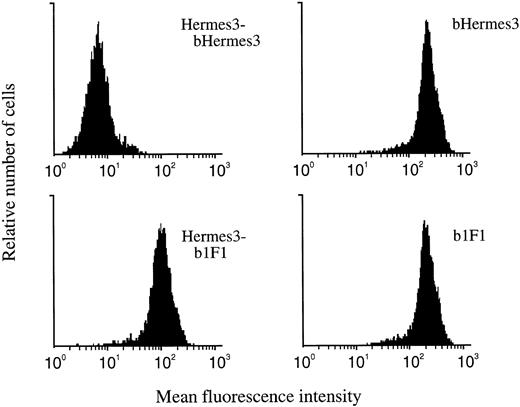
Biotinylated 1F1 incubated after Hermes-3 antibody still strongly stained CD44 transfectants, indicating that MoAb 1F1 recognizes a different epitope in the constant part of CD44 than MoAb Hermes-3. The combination of Hermes-3 and biotinylated Hermes-3 was used as a negative control (Fig 1).
The epitope mapping. MoAb 1F1 recognizes CD44 transfected cells after incubation with MoAb Hermes-3. Combination of Hermes-3–biotinylated Hermes-3 is used as the negative control. b, biotinylated.
The epitope mapping. MoAb 1F1 recognizes CD44 transfected cells after incubation with MoAb Hermes-3. Combination of Hermes-3–biotinylated Hermes-3 is used as the negative control. b, biotinylated.
Immunohistochemical staining. Five-micrometer frozen tonsil sections were cut, air-dried, and acetone-fixed. The sections were overlaid with hybridoma supernatant and incubated for 30 minutes at room temperature in a humidified chamber. After two washes in phosphate-buffered saline (PBS), peroxidase-conjugated rabbit antimouse Ig (DAKO A/S, Glostrup, Denmark) was added. Reaction was developed by adding 3,3-diaminobenzidine (Polysciences, Inc, Warrington, PA) in PBS containing 0.03% hydrogen peroxide for 3 minutes. After staining, the sections were dehydrated, cleared in xylene, and permanently mounted in DePex (BDH Ltd, Poole, Dorset, UK).
Chemiluminescence-EIA. White streptavidin-coated 96-well EIA-plates (White Combiplate 8 Streptavidin Coated; Labsystems, Helsinki, Finland) were incubated with biotinylated Hermes-3 for 60 minutes at room temperature (RT). The biotinylation was performed using a standard procedure with biotin-NHS (Calbiochem, Calbiochem-Novobiochem Corp, La Jolla, CA). Nonspecific binding sites were blocked by incubation with PBS containing 1% dried nonfat milk powder and 1% gelatin for 45 minutes. Diluted serum samples were added and incubated for 60 minutes. The detecting MoAb 1F1 was added and incubated for 60 minutes. Peroxidase-conjugated monoclonal rat antimouse IgG2b (Zymed Laboratories, Inc, San Francisco, CA) was used as the second-stage antibody. Between incubations, the wells were washed extensively with PBS containing 0.3% Tween-20. Immunodetection was performed using BM Chemiluminescence ELISA Reagents (Boeringer Mannheim GmbH, Mannheim, Germany). The degree of luminescence was quantified using a luminometer (Luminoscan; Labsystems). CD44-depleted serum was used as a negative control serum. Soluble CD44 was removed from serum by incubation with Hermes-3 coupled to CNBr-activated Sepharose 4B beads (Pharmacia, Uppsala, Sweden). Serum with a high concentration of CD44 and tonsil lysates with known concentrations of soluble CD44 were used as positive controls.
In each EIA assay, CD44-depleted serum was added as a negative control. The minimal detectable concentration, defined as +2 standard deviations (SD) above the value of the negative control, was 4.9 ng/mL. s-CD44 concentrations of all patient samples were on the linear part of the titration curve and each sample was tested in triplicate. Interassay and intra-assay variations were both less than 10%.
In the present series, s-LDH measurements were performed at the time of the diagnosis, unlike the s-CD44 measurements that were performed after storing the sera for 9 to 15 years. Hypothetically, the long storage of the serum samples before analysis might have influenced the s-CD44 levels, and, thus, a better correlation with s-CD44 levels and prognosis might have been obtained if s-CD44 determinations could have been performed prospectively. To study the effect of freezing on the measured s-CD44 levels, we first measured s-CD44 concentrations in freshly taken serum samples and after freezing and melting the same samples several times. In these experiments, we found similar s-CD44 levels before and after freezing, suggesting that CD44 is not easily degraded, perhaps due to its heavy glycosylation.
Statistical analysis. Statistical analyses were performed with the BMDP computer program (BMDP Statistical Software, Department of Biomathematics, University of California, Los Angeles, CA). Frequency tables were analyzed with the χ2 test or Fisher's exact test. s-CD44 distributions between different groups were analyzed with the Mann-Whitney's test. Cumulative survival between groups was compared with the log-rank test. The relative importance of different variables was analyzed using Cox's proportional hazard model (BMDP 2L). All P values are two-tailed.
RESULTS
Association of s-CD44 with clinical factors. The median s-CD44 concentration measured at diagnosis in patients with NHL (n = 194) was 440 ng/mL (range, 13 to 1,220 ng/mL). Associations between s-CD44 and 8 clinicopathologic factors are shown in Table 1. A low CD44 level was strongly associated with a small Ann Arbor stage (P < .0001), normal serum LDH at diagnosis (P = .0005), absence of B symptoms (P = .003), age less than 60 years at diagnosis (P = .02), good World Health Organization (WHO) performance status (P = .02), and a limited number (0 or 1) of extranodal sites (P = .05). Seventy-nine (62%) of the 127 patients who achieved a complete remission had an s-CD44 level lower than the median at the time of diagnosis, whereas only 14 (30%) of the 46 patients who did not achieve a complete response had an s-CD44 lower than the median (P = .0002).
In the largest single subgroup of DLCL (n = 51), the median s-CD44 level was 493 ng/mL (range, 15 to 1,076 ng/mL). Again, a low CD44 level turned out to be strongly associated with a small Ann Arbor stage (P = .0007), good WHO performance status (P = .005), and a normal LDH level at diagnosis (P = .009, Table 2). Forty-seven patients with DLCL were evaluable for response to therapy, and 41 (87%) achieved a complete remission and 6 (13%) a partial remission. Twenty-five (61%) of the 41 patients who achieved a complete remission had an s-CD44 level at diagnosis lower than the median (493 ng/mL), whereas 5 (83%) of the 6 patients who achieved only a partial remission had a s-CD44 level higher than the median (P = .08).
Correlation with the IPI. The IPI was available for 183 patients. The patients with a high IPI score (≥2) had significantly higher s-CD44 concentrations than those with a low IPI score (0 or 1) when the median (440 ng/mL) was used as the cut-off value (P < .0001). Only 29 (32%) of the 92 patients with an IPI score 0 or 1 had s-CD44 concentration higher than the median level, whereas 61 (67%) of 91 patients with an IPI score ≥2 had a concentration higher than the median at diagnosis (P < .0001, Table 3).
In DLCL, the IPI score was available for 48 patients, of whom 27 (56%) had a low score (0 or 1) and 21 (44%) had a higher risk disease (score ≥2). Patients with a score of 0 or 1 had lower s-CD44 concentrations at the time of the diagnosis than those with a score ≥2. Twenty-two (81%) of the 27 patients with a low IPI score (0 or 1) had an s-CD44 concentration lower than the median value (493 ng/mL), whereas only 3 (14%) of the 21 patients with a high IPI score (≥2) had an s-CD44 level lower than the median at diagnosis (P < .0001). We investigated the association between the s-CD44 level and the IPI score also among all patients who had lymphoma of some other type than DLCL (n = 143, the IPI score was available in 135 cases) and found a higher than the median s-CD44 level (422 ng/mL) to be associated with a high IPI score in this subgroup, too (P = .01). In the subgroup of low-grade lymphomas, the median s-CD44 level was 432 ng/mL (range, 63 to 983 ng/mL), and the IPI score was available for 46 patients. In these patients, low s-CD44 level was not associated with a low IPI score (the median was used as the cut-off value, P = .67).
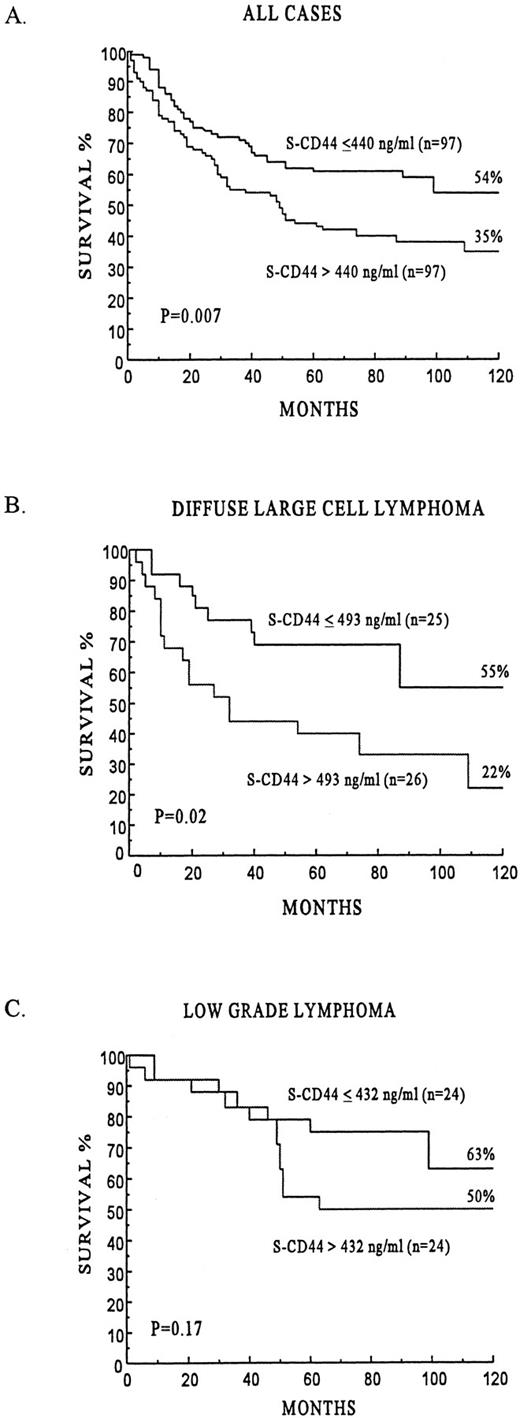
Survival. The univariate survival analyses by the IPI score, the factors used to construct the IPI, B symptoms, gender, and s-CD44 are shown in Table 4. Patients with a higher than the median s-CD44 concentration at the time of the diagnosis had poorer survival than those with lower s-CD44 concentrations. The 5- and 10-year survival rates of the subgroup with higher than the median s-CD44 level were 44% and 35%, respectively, whereas the corresponding figures for patients with lower than the median s-CD44 levels were 62% and 54%, respectively (P = .007, Fig 2A). Similarly, the subgroup of DLCL patients with a high s-CD44 level had inferior survival as compared with those with a low s-CD44 level (the median was used as the cut-off value, P = .02, Fig 2B). A higher than the median s-CD44 level was associated with poor survival also among patients who had some other type of lymphoma than DLCL (n = 143; 5-year survival rate, 44% v 61%; P = .04). In patients with low-grade lymphoma, s-CD44 level was not significantly associated with survival (P = .17, Fig 2C).
The prognostic influence of s-CD44 in all lymphomas and separately in the subgroups of DLCL and low-grade lymphomas.
The prognostic influence of s-CD44 in all lymphomas and separately in the subgroups of DLCL and low-grade lymphomas.
In a multivariate analysis, the IPI score had independent prognostic value (P < .001; relative risk of death, 2.2; 95% confidence interval [CI], 1.7 to 2.7) and the presence of B symptoms was possibly an independent prognostic factor (P = .06; relative risk, 1.6; 95% CI, 1.0 to 2.5), but s-CD44 did not have independent influence on survival. When the weakest single factor in a univariate survival analysis of the prognostic variables included in the IPI, the number of involved extranodal sites, was replaced in the IPI by s-CD44 using either the median or the highest tertile as the cut-off value, the prognostic significance of the IPI was reduced slightly also, suggesting that s-CD44 does not add to the prognostic value of the IPI. Similarly, as in the entire series, in DLCL the IPI score turned out to be the only variable with independent influence on survival in a multivariate analysis (P < .001; relative risk of death, 3.7; 95% CI, 2.3 to 5.8).
DISCUSSION
In the present study, s-CD44 concentration measured using a novel chemiluminescence-EIA method correlated well with the IPI score, and high-risk patients often had a high s-CD44 level at the time of the diagnosis. The s-CD44 level was also associated with response to treatment, and patients with a low pretreatment s-CD44 level achieved more often a complete remission. A high pretreatment s-CD44 level was associated with poor survival both among unselected patients with NHL and in the subgroup of DLCL when treatment was usually based on a doxorubicin-containing combination regimen. Interestingly, in the subgroup of patients with low-grade lymphoma, the pretreatment s-CD44 level did not have prognostic value. A high s-CD44 level was particularly strongly associated with a high Ann Arbor stage and a high s-LDH level, but not with the Working Formulation grade. Seven (63%) of the 11 cases of small-cell lymphocytic lymphomas but only 10 (43%) of the 23 immunoblastic lymphomas in the present series had an s-CD44 level higher than 440 ng/mL, which also suggests that s-CD44 levels correlate with lymphoma dissemination or possibly with the tumor burden rather than with the aggressiveness of lymphoma.
s-CD44 was found for the first time in the serum in 1980.30 Since then, there have been only few studies concerning the functions and clinical importance of circulating CD44, and both have been poorly understood. Although s-CD44 lacks the cytoplasmic domain of the molecule, it still has biologic activity in functional studies.24,26 In vitro, s-CD44 is able to bind components of the extracellular matrix, such as hyaluronate and fibronectin. s-CD44 may also have importance as a mechanism against hematogenous dissemination of lymphoma, because s-CD44 is able to block the binding of peripheral blood lymphocytes and probably also lymphoma cells to the high endothelial venules.24 CD44 may also influence cell proliferation, because certain forms of CD44 are able to bind growth factors. However, these suggested functions are still largely speculative.
We have earlier found that serum CD44 levels change in parallel with treatment response in malignant lymphoma.22 s-CD44 concentrations measured before lymphoma treatment and in progressive disease were clearly higher than those found at complete remission or in healthy individuals without lymphoma. In this respect, CD44 may be superior to more often used lymphoma serum markers, s-LDH and thymidine kinase,31,32 but this needs to be investigated in a prospective study. Both s-LDH and thymidine kinase have prognostic value in NHL, but they are not reliable indicators of treatment response, because chemotherapy or hematopoietic growth factors may increase the serum levels of LDH and thymidine kinase despite good response to treatment.33
The origin of soluble CD44 has not been firmly established, but at least a part of s-CD44 in cancer patients probably originates from tumor cells. In patients with gastric or colon carcinoma, the molecular mass of s-CD44 is predominantly 130 to 190 kD, which reflects the large size of CD44 found in carcinoma cells.23 Both in lymphoma cells and in normal hematopoietic cells, the molecular mass of CD44 is usually 90 kD, and, thus, the origin of s-CD44 in lymphoma patients cannot be deduced simply on the basis of the molecular mass.24 However, the strong association between s-CD44 levels and stage, change of CD44 in parallel with treatment response,22 and gene transfection studies24 all suggest that in lymphoma, too, the origin of s-CD44 is at least partly in lymphoma deposits.
In conclusion, a high pretreatment s-CD44 level is associated with a high Ann Arbor stage and higher than normal s-LDH concentration, poor response to treatment, and poor survival both in NHL and in the subgroup of DLCL. However, a significant association with the IPI score or survival was not found among patients with low-grade lymphoma, and the IPI was the only independent prognostic factor in multivariate analyses both in the entire series and in the subset of DLCL. The function of soluble CD44 is not well understood, but s-CD44 probably originates partially from lymphoma deposits. More data need to be collected to establish the clinical and prognostic significance of s-CD44 in other subtypes of NHL than DLCL and in Hodgkin's disease.
Supported by grants from the Finnish Cancer Foundation, the Maud Kuistila Foundation, the Nordic Cancer Research Council, the Finnish Medical Foundation, the Finnish Academy, and the Ida Montin Foundation.
Address reprint requests to Raija Ristamäki, MD, MediCity Research Laboratory, Turku University, Tykistökatu 6 A, 20520 Turku, Finland.