Abstract
The effectiveness of thrombopoietin (TPO) in alleviating thrombocytopenia was evaluated in a placebo-controlled study involving rhesus monkeys exposed to 5 Gy total-body irradiation (TBI) (300-kV x-rays) to result in 3 weeks of pancytopenia. Supraoptimal treatment with human recombinant TPO (10 μg/kg/d subcutaneously, days 1 to 21 after TBI) was highly effective in preventing thrombocytopenia, with nadirs for thrombocytes, on average, far higher than 100 × 109/L, a greatly accelerated recovery to normal values, and no need for thrombocyte transfusions. TPO appeared to act selectively in that neutrophil regeneration was not influenced but red blood cell lineage recovery was prominently stimulated, with reticulocyte regeneration being initiated 10 days earlier than in placebo-treated animals. The reticulocytosis was followed by a normoblastosis that occurred earlier and was more pronounced than in placebo-treated monkeys. The effect of TPO on the red blood cell lineage was also reflected in a less profound nadir for hemoglobin (Hb) and hematocrit values than in placebo controls. However, this effect was not followed by a rapid recovery to normal values, due to development of a microcytic hypochromic anemia. Iron depletion was demonstrated by measurements of total serum iron and total iron-binding capacity (TIBC) and could be prevented by prophylactic intramuscular (IM) iron before TBI or corrected by IM iron after TPO treatment. Rechallenging with TPO in week 8 after TBI demonstrated a homogenous thrombocyte response similar in magnitude to the initial response, but a greatly diminished reticulocyte response. This demonstrated that the erythropoietic response to TPO administration depends on the hemopoietic state of the animal and may reflect multiple TPO target cells. It is postulated that the extremely rapid erythropoiesis due to TPO treatment in the initial regeneration phase following myelosuppression results in iron depletion by a mechanism similar to that seen following erythropoietin treatment in patients with end-stage renal failure. It is concluded that protracted TPO therapy to counteract thrombocytopenic states may result in iron depletion and that the iron status should be monitored before, during, and after TPO treatment.
THROMBOPOIETIN (TPO), the ligand for c-mpl, was identified and its gene cloned in 1994.1-3 In vitro and in vivo evaluation showed it to be the major regulator of thrombocyte production.4-7 In vitro experiments also demonstrated, apart from megakaryocyte colony formation,6,8,9 effects on erythroid progenitors in synergy with erythropoietin.10,11 In normal experimental animals, thrombocyte counts increased to supranormal values without affecting other lineages.3,7,12-15 In several myelosuppression models, TPO has also been found to stimulate, apart from the megakaryocytic lineage, erythroid precursors,13,14,16,17 expansion of immature bone marrow progenitor cells,18 and, to a lesser extent granulocyte-macrophage colony-forming units.16 17 Adverse effects have not been observed. In the present study, TPO was evaluated in a rhesus monkey model for myelosuppression to investigate its ability to alleviate thrombocytopenia. The monkeys were subjected to 5 Gy x-ray total-body irradiation (TBI) resulting in 3 weeks of profound pancytopenia. We report here that TPO, apart from greatly promoting thrombocyte regeneration after myelosuppression, also stimulated erythropoiesis, leading to iron depletion and resulting in microcytic anemia.
MATERIALS AND METHODS
Animals.Purpose-bred male rhesus monkeys (Macaca mulatta) weighing 2.5 to 4.0 kg and aged 2 to 3 years were used. The monkeys were housed in groups of four to six in stainless steel cages in rooms with a reverse-filtered air barrier and normal daylight rhythm and conditioned to 20°C with a relative humidity of 70%. Animals were fed ad libitum with commercial primate chow and fresh fruits and received acidified drinking water. All animals were free of intestinal parasites and were seronegative for herpes B, simian T-lymphotrophic viruses and simian immunodeficiency virus. The housing, experiments, and all other conditions were approved by an ethics committee in accordance with legal regulations in The Netherlands.
TBI.Monkeys were irradiated with a single dose of 5 Gy TBI delivered by two opposing x-ray generators operating at a tube voltage of 300 kV and a current of 10 mA. The half-layer thickness was 3 mm Cu. The focus skin distance was 0.8 m, and the mean dose rate 0.20 to 0.22 Gy/min. During TBI, the animals were placed in a cylindrical polycarbonate cage that rotated slowly (three times per minute) around its vertical axis.
Experimental procedure.Two weeks before TBI, the monkeys were placed in a laminar-flow cabinet, and the gastrointestinal tract was selectively decontaminated by giving oral Ciproxin (Bayer, Mijdrecht, The Netherlands), nystatin (Sanofi, Maassluis, The Netherlands), and polymyxin B (Pfizer, New York, NY). This regimen was maintained until leukocyte counts exceeded 109/L. Systemic antibiotics were given when leukocyte counts were less than 109/L, in most cases as a combination of Ticarpen (Beecham Pharma, Amstelveen, The Netherlands) and Zinacef (Glaxo, Zeist, The Netherlands), as guided by fecal bacteriograms. Dehydration and electrolyte disturbances were treated by appropriate fluid and electrolyte administration subcutaneously. The monkeys received irradiated (15 Gy) thrombocyte transfusions whenever thrombocyte counts were less than 40 × 109/L and irradiated packed red blood cells whenever hematocrits were less than 20%, and occasionally, the monkeys received whole blood transfusions in case of simultaneous occurrence of both transfusion criteria. All monkeys were bled for diagnostic purposes, 7 mL before irradiation, 7 mL twice weekly during the first 4 weeks, and 3 mL once weekly thereafter. Three milliliters of bone marrow was aspirated before irradiation and once weekly for 6 weeks after TBI. The total blood volume of 3-kg monkeys is estimated to be 200 mL.
Test drug.One milliliter vials containing 0.5 mg/mL recombinant full-length human TPO produced by CHO cells were supplied by Genentech Inc (South San Francisco, CA). The dose used was 10 μg/kg/d subcutaneously once daily from day 1 to day 21 after irradiation (n = 4). Three monkeys received the same dose for a second period of 7 days from day 52 to day 58 after irradiation. The daily doses were diluted to a volume of 1 mL with phosphate-buffered saline (PBS)/0.01% Tween 80. Placebo-treated monkeys were only given the same volume of diluent (n = 4).
Hematologic examinations.Complete blood cell counts were measured daily using a Sysmex F-800 hematology analyzer (Toa Medical Electronics Co, Kobe, Japan). The differential of the total nucleated cells was determined by standard counting after May-Grünwald-Giemsa staining. For reticulocyte measurements, 5 μL EDTA blood was diluted in 1 mL PBS/EDTA (5.0 mol/L)/azide (0.05% wt/vol) and 1 mL of a thiazole orange dilution was added, using thiazole in a final concentration of 0.5 μg/mL. Measurements were made on a FACScan (Becton Dickinson, San Jose, CA) and analyzed using the Reticount software (Becton Dickinson).
Iron administration.Three monkeys received iron supplementation. One monkey received 0.5 mL Imferon (Fe(III) 50 mg/mL; Fisons Pharmaceuticals, Loughborough, England) for 5 consecutive days before irradiation, and two monkeys received 3 mL in 10 days approximately 3 months after irradiation. Imferon was given intramuscularly.
Clinical chemistry.Serum levels of sodium, potassium, chloride, glucose, albumin, total protein, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase isotype 1 (LDH1), γ-glutamyl transferase, total bilirubin, C-reactive protein, creatinine, urea, and CO2 were measured twice weekly using an Elan Analyzer (Eppendorf Merck, Hamburg, Germany). Total serum iron and total iron-binding capacity (TIBC) were determined in representative stored serum samples, and the percentage saturation was calculated.
Postmortem material.As arranged with the ethics committee for animal experiments operating at our university, all animals were euthanized at the end of the study. Most of the animals were euthanized at day 43 after irradiation by injecting 2 mL Euthasate (Apharmo, Arnhem, The Netherlands; pentobarbital 200 mg/mL) intravenously. For two of the TPO-treated monkeys, the follow-up time was prolonged to further evaluate the anemia, and for one placebo-treated monkey for other reasons. At the time of obduction, organs were fixed with 4% paraformaldehyde, and about 24 hours later the material was embedded in paraffin and slides were made stained with hematoxylin/eosin. Slides for iron staining were made from the paraffin-embedded material.
Statistics.If relevant, the mean ± SD was calculated with the assumption of a normal distribution. The significance of a difference was calculated by one-way analysis of variance followed by a Student's t-test.
RESULTS
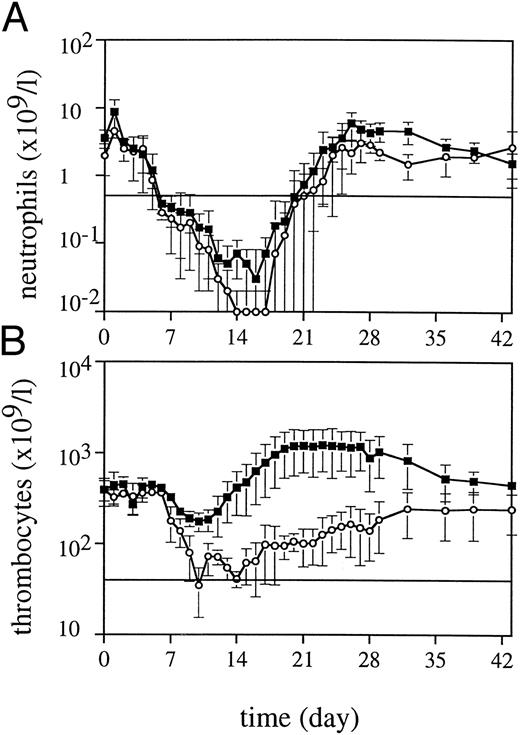
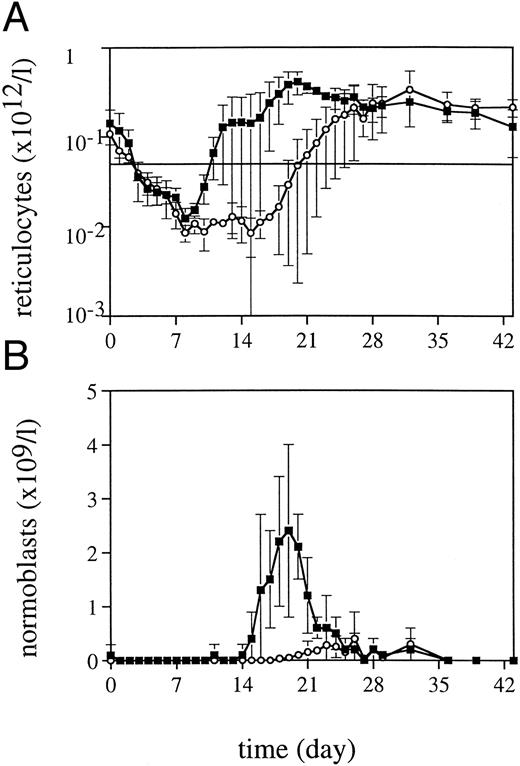
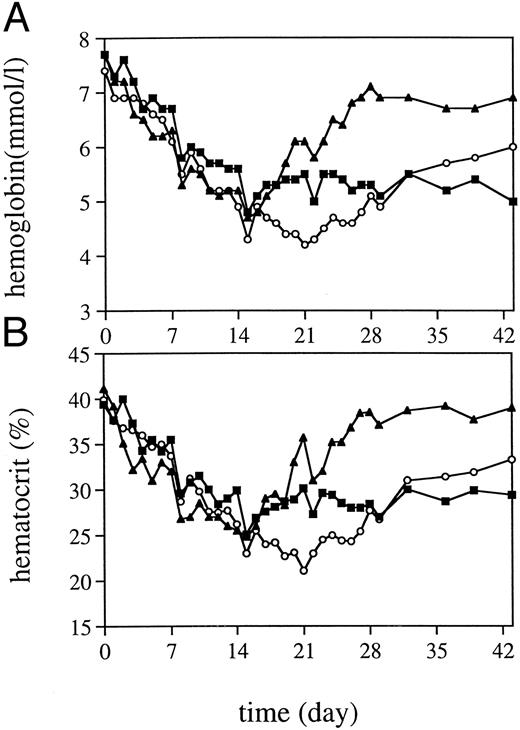
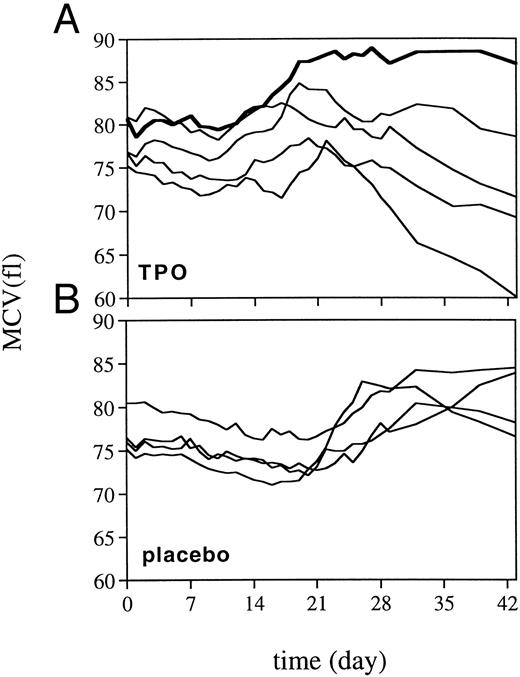
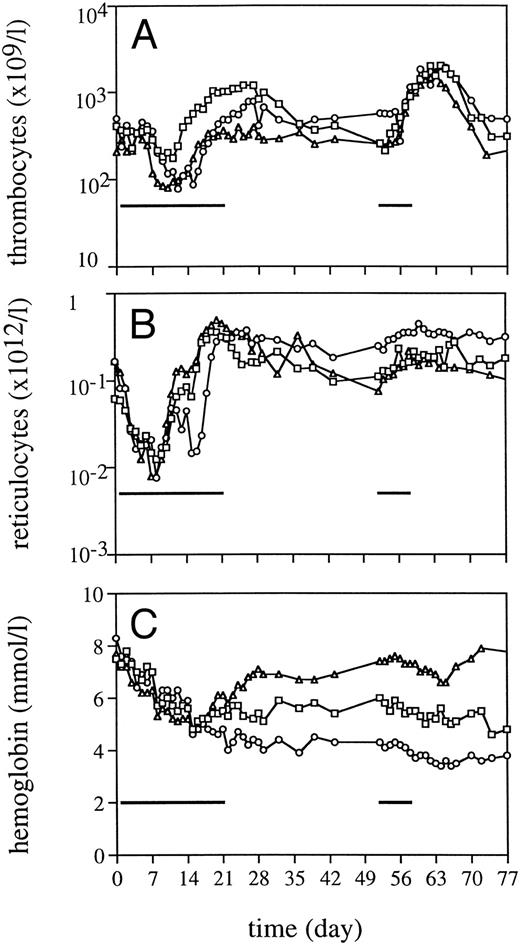
Peripheral blood cell counts.TPO was highly effective in alleviating thrombocytopenia after irradiation, and recovery to normal values was significantly accelerated (Fig 1). The dose schedule of TPO was supraoptimal, as is clear from the increase to supranormal thrombocyte counts in the third week of treatment. The kinetics of the thrombocyte response strongly suggests that TPO treatment during the first week after TBI would have been sufficient for prevention of thrombocytopenia. One week after cessation of TPO treatment, thrombocyte counts gradually returned to normal. TPO treatment was selectively effective in that neutrophil regeneration was not influenced (Fig 1), with values more than 0.5 × 109/L being reached at day 20 (range, 20 to 23). In addition, TPO had a prominent effect on red blood cell parameters. Reticulocyte regeneration, on average, was 10 days earlier than in placebo-treated controls, and TPO-treated monkeys developed a normoblastosis that was also earlier and much more pronounced (Fig 2). All monkeys developed anemia, partly due to diagnostic bleeding. The decrease in hemoglobin (Hb) concentration was similar in all monkeys until day 14 after TBI (Fig 3). TPO-treated monkeys showed less profound nadirs than placebo-treated controls, and Hb levels stabilized after the second week. However, the earlier stabilization of Hb levels was not reflected in an earlier recovery to normal values. Instead, TPO-treated monkeys developed a sustained anemia, whereas placebo-treated monkeys were able to recover to subnormal Hb levels at the end of the observation period (6 weeks) (Fig 3). The anemia of TPO-treated monkeys was microcytic (Fig 4) and hypochromic (Table 1) in nature. The difference in mean cell volume (MCV) kinetics is most apparent if the data set of each monkey is shown individually. The MCV of TPO-treated monkeys showed an initial increase that coincided with the reticulocyte recovery and was followed by a gradual decline in the fifth and sixth weeks of treatment, whereas placebo-treated monkeys all had an increase in MCV values from the third week onward, also coinciding with reticulocyte recovery. Mean cellular Hb (MCH) was also decreased in TPO-treated monkeys in the sixth week after TBI compared with pretreatment values, whereas in placebo-treated monkeys MCH values remained constant (Table 1).
Neutrophil (A) and thrombocyte (B) regeneration after 5 Gy TBI for TPO-treated monkeys (▪, n = 4) and placebo-treated controls (○, n = 4).
Neutrophil (A) and thrombocyte (B) regeneration after 5 Gy TBI for TPO-treated monkeys (▪, n = 4) and placebo-treated controls (○, n = 4).
Reticulocyte regeneration (A) and the appearance of normoblasts (B) after 5 Gy TBI for TPO-treated monkeys (▪, n = 4) and placebo-treated controls (○, n = 4).
Reticulocyte regeneration (A) and the appearance of normoblasts (B) after 5 Gy TBI for TPO-treated monkeys (▪, n = 4) and placebo-treated controls (○, n = 4).
Hb levels (A) and hematocrit (B) after 5 Gy TBI for TPO-treated monkeys (▪, n = 4), placebo-treated controls (○, n = 4), and one iron-pretreated TPO-treated monkey (▴).
Hb levels (A) and hematocrit (B) after 5 Gy TBI for TPO-treated monkeys (▪, n = 4), placebo-treated controls (○, n = 4), and one iron-pretreated TPO-treated monkey (▴).
MCV after 5 Gy TBI for TPO-treated monkeys (A) and placebo-treated controls (B). Each line represents an individual monkey: bold line, iron-pretreated TPO-treated monkey.
MCV after 5 Gy TBI for TPO-treated monkeys (A) and placebo-treated controls (B). Each line represents an individual monkey: bold line, iron-pretreated TPO-treated monkey.
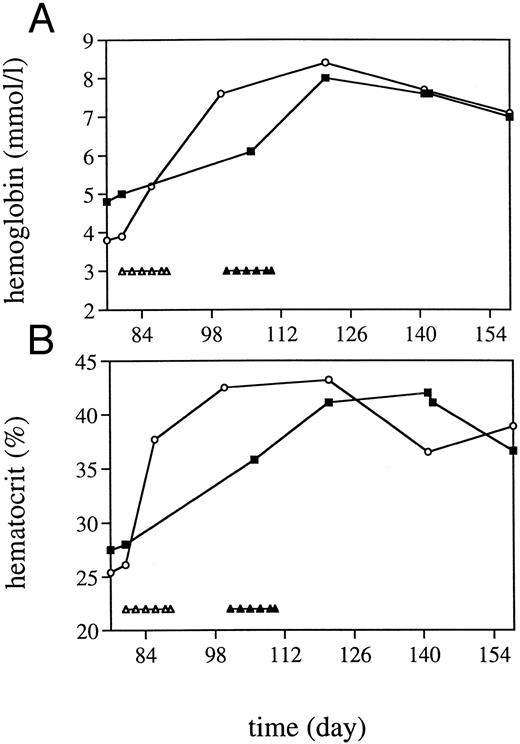
TPO rechallenge and iron supplementation.To examine the effectiveness of TPO after myelosuppression without limited iron supplies, one monkey was given intramuscular (IM) iron during the week before TBI, followed by TPO treatment. The nadir for Hb and hematocrit was similar to those of the four monkeys treated with TPO earlier, but the iron-treated monkey had a regeneration of Hb levels much earlier than the other TPO-treated monkeys and the placebo controls (Fig 3) and did not develop a microcytic anemia. Thrombocyte regeneration of the iron-treated monkey was in the range of that for the four monkeys treated earlier (Fig 5). In an extended follow-up period, two TPO-treated monkeys and the single TPO/iron-treated monkey were rechallenged with TPO in week 8 after TBI, after which a thrombocyte production response similar to that reported for normal nonhuman primates occurred15 (Fig 5). All monkeys had a small but significant increase in reticulocyte counts, which only in the iron-treated monkey was followed by a small increase in Hb levels after an initial decline due to diagnostic bleeding. In the other two monkeys, Hb levels stabilized. Subsequent IM iron administered to the latter animals over a period of 10 days in the third month after TBI resulted in a prompt increase of Hb and hematocrit levels (Fig 6) and a correction of anemia.
Effect of a rechallenge with TPO (10 μg/kg/d) from days 52 to 58 in three monkeys on thrombocytes (A), reticulocytes (B), and Hb (C). Two TPO-treated monkeys (□, ○) and one iron-pretreated, TPO-treated monkey (▵). Black lines, duration of TPO treatment.
Effect of a rechallenge with TPO (10 μg/kg/d) from days 52 to 58 in three monkeys on thrombocytes (A), reticulocytes (B), and Hb (C). Two TPO-treated monkeys (□, ○) and one iron-pretreated, TPO-treated monkey (▵). Black lines, duration of TPO treatment.
Effect of iron treatment in two anemic TPO-treated monkeys. (A) Hb level; (B) hematocrit. Iron was administered at the indicated time points. (▵) Correspond with the monkey represented with (○), and (▴) with (▪). At the indicated time points, 30 mg Fe(III) was administered.
Effect of iron treatment in two anemic TPO-treated monkeys. (A) Hb level; (B) hematocrit. Iron was administered at the indicated time points. (▵) Correspond with the monkey represented with (○), and (▴) with (▪). At the indicated time points, 30 mg Fe(III) was administered.
Clinical parameters and transfusion requirements.The number of febrile episodes was not different between the two groups. Normal axillary body temperature in rhesus monkeys is 39°C, and fever is defined as a morning temperature more than 40°C. TPO-treated monkeys had 3.7 ± 2.9 days with fever, and placebo-treated controls 4.5 ± 3.5 days, statistically nonsignificant (P = .76).
Since iron contained in blood transfusions might confound our results, the transfusion requirement of the monkeys is also presented. TPO-treated monkeys did not receive any blood products during the experiment. All placebo-treated monkeys received several thrombocyte transfusions, which are not considered to contain significant amounts of iron. Three of four placebo-treated controls received a whole blood transfusion at days 15, 21, and 26 after TBI, respectively. This is too late in the time course to account for the difference in the Hb nadir occurring in the third week after TBI.
Clinical chemistry.TPO-treated animals showed increased serum LDH1 levels due to the rapid but insufficient erythropoiesis. However, the iron-pretreated monkey did not display this elevation in LDH1 levels, and the normoblastosis seen in TPO-treated monkeys was also reduced in this monkey (data not shown).
To assess the iron status of the monkeys before and during therapy, total serum iron and TIBC of representative stored serum samples were determined. Iron saturation was 27% ± 12% before irradiation, but decreased to much lower levels 6 weeks after irradiation (6% ± 1% for TPO, P = .04; 17% ± 8% for placebo, nonsignificant). The difference between TPO- and placebo-treated monkeys at week 6 is also significant (P < .03; Table 1). Serum iron followed a similar pattern in that at day 14 free serum iron was higher, probably due to diminished iron utilization in the initial period after TBI during which erythropoiesis was profoundly suppressed (Table 1). In the iron-pretreated monkey, total serum iron also decreased steadily after TBI, but not to a degree sufficiently severe to induce anemia. In TPO- and placebo-treated monkeys, iron stores were not detectable in bone marrow slides stained for iron (data not shown).
DISCUSSION
TPO was effective in stimulating thrombocyte recovery after a myelosuppressive exposure to TBI and fully prevented the development of thrombocytopenia. An effect on neutrophil regeneration was not observed. TPO also prominently stimulated the erythroid lineage, an effect that occurred very early after irradiation: exponential reticulocyte regeneration started after the first week of treatment, 10 days earlier than in placebo-treated controls. The reticulocytosis was followed by a normoblastosis accompanied by elevated LDH serum levels, attributed to the rapid and probably inefficient erythropoiesis. As a result, Hb levels and hematocrits stabilized earlier at higher levels. However, in TPO-treated monkeys, signs of a microcytic hypochromic anemia became apparent after the third week, suggesting depletion of iron stores. In this study, the development of iron deficiency during TPO treatment was directly confirmed, as was its correction by IM iron treatment and prevention by prophylactic IM iron.
The kinetics of serum iron and TIBC displayed an initial increase during the 2 weeks after TBI, which is explained by a lack of iron utilization due to myelosuppression. This increase is particularly prominent in the placebo-treated monkeys, but is also statistically significant in the TPO-treated monkeys (Table 1). The smaller increase of serum iron measured on day 14 after TPO compared with the placebo control levels is attributed to the early onset of erythropoietic reconstitution in TPO-treated monkeys. The subsequent decline in serum iron and serum iron/TIBC is larger in TPO-treated monkeys, resulting in very low levels and a state of iron deficiency at the end of the observation period of 6 weeks (Table 1) in TPO-treated monkeys. It is not excluded that the iron deficiency essentially originated from a precarious iron balance in rhesus monkeys, similar to humans, resulting in latent iron deficiency already present before radiation exposure. This is more likely, since the MCV in the animals before irradiation was 75 to 80 and increased to 90 in the animal subjected to iron supplementation. The latent iron deficiency may then be caused by diet, malabsorption due to the preirradiation antibiotic regimen, or some other process related to iron absorption.
Three TPO-treated monkeys, two without iron supplementation and one provided with prophylactic IM iron before TBI, were rechallenged with TPO in week 8 after TBI after full recovery from myelosuppression, and showed a homogeneous stimulation of thrombocyte production. This indicated that (1) the effect of TPO on thrombocytes is independent of iron status, since only the iron-pretreated monkey had a demonstrated normal iron status at that time point, and (2) the variation of the TPO response following TBI should not be attributed to individual sensitivity of the animals to TPO. Rather, this should be attributed to a variation in the number of residual TPO target cells following TBI, possibly due to individual sensitivity to radiation. The rechallenge experiments also demonstrated a differential reticulocyte response to TPO early and late after TBI. Although the platelet responses were similar in magnitude, reticulocyte responses were much diminished upon rechallenge (Fig 5). This may reflect either multiple target cells of TPO and/or involvement of growth factors other than TPO in the reticulocyte response. The magnitude of reticulocytosis upon rechallenge was roughly inversely proportional to the Hb level, indicating an effect codependent on erythropoietin.
Iron supplementation to two anemic monkeys proved to be effective in treating anemia. Iron prophylaxis before TBI completely prevented microcytic anemia and Hb levels quickly returned to normal, further demonstrating the pronounced effect of TPO on red blood cell reconstitution in the initial phase after TBI.
Multilineage effects, including the erythroid lineage, of TPO have been shown in vitro and in vivo.10,11,16,18 In normal mice and primates, TPO selectively stimulated thrombocyte production, leaving other lineages unaffected. In myelosuppression models, multilineage effects were mainly of an erythroid nature.14,16,19 Since megakaryocytes are capable of releasing various growth factors,20,21 the effect observed may be an indirect consequence of stimulation of the megakaryocytic lineage. This does not exclude a direct effect of TPO, since erythroid progenitors have been shown in vitro to be directly stimulated by TPO.11 The iron depletion of TPO-treated monkeys is reminiscent of erythropoietin-induced iron deficiency anemia in patients with end-stage renal failure.22-24 A similar mechanism is proposed for the iron deficiency anemia occurring in the present study. Supplementation of iron before and careful monitoring during treatment proved to be necessary for all patients receiving erythropoietin. It is concluded that TPO therapy to counteract thrombocytopenic states is highly effective to prevent thrombocytopenia, and that its beneficial effect on erythroid regeneration may be hampered by iron depletion. Therefore, the iron status should be carefully monitored in case of therapeutic TPO administration.
ACKNOWLEDGMENT
The authors wish to thank Trudy Visser, Hannie Busking-van der Lelie, Dorinde Kieboom-Pluimes, and Pim van Schalkwijk for technical assistance, and Dr Aad van der Lugt for critical reading of the manuscript.
Supported by The Netherlands Cancer Foundation Koningin Wilhelmina Fonds, the Dutch Organization for Scientific Research NWO, the Royal Netherlands Academy of Arts and Sciences, and Contracts of the Commission of the European Communities.
Address reprint requests to Gerard Wagemaker, PhD, Institute of Hematology, H Ee 1314, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands.