Abstract
Plasminogen activator inhibitor-1 (PAI-1), the primary inhibitor of tissue- and urokinase-type plasminogen activators, is considered a critical regulator of the fibrinolytic system. We previously reported a child with abnormal bleeding and complete PAI-1 deficiency caused by a frame-shift mutation in exon 4 of the PAI-1 gene. The purpose of this study was to provide genetic and clinical data on the extended pedigree of the original proband to better define the phenotype associated with PAI-1 deficiency. Allele-specific oligonucleotide hybridization was used to genotype individuals, and serum PAI-1 antigen was measured by enzyme-linked immunosorbent assay. By this approach we have identified 19 individuals who are heterozygous for the PAI-1 null allele and 7 homozygous individuals with complete PAI-1 deficiency. Clinical manifestations of PAI-1 deficiency were restricted to abnormal bleeding, which was observed only after trauma or surgery in homozygous affected individuals. A spectrum of bleeding patterns was observed, including intracranial and joint bleeding after mild trauma, delayed surgical bleeding, severe menstrual bleeding, and frequent bruising. Fibrinolysis inhibitors, including ε-aminocaproic acid and tranexamic acid, were effective in treating and preventing bleeding episodes. Other than abnormal bleeding, no significant developmental or other abnormalities were observed in homozygous PAI-1–deficient individuals. Heterozygous PAI-1 deficiency was not associated with abnormal bleeding, even after trauma or surgery. These observations define the clinical spectrum of PAI-1 deficiency and provide additional evidence to support the hypothesis that the primary function of plasminogen activator inhibitor-1 in vivo is to regulate vascular fibrinolysis.
PLASMINOGEN ACTIVATOR inhibitor-1 (PAI-1) is a fast-acting inhibitor of tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA).1,2 PAI-1 is a member of the serpin superfamily of protease inhibitors.3 Like other serpins, PAI-1 reacts with its cognate proteases to form 1:1 enzyme-inhibitor complexes that are enzymatically inactive. PAI-1 is the primary inhibitor of t-PA in plasma.1 Platelets, vascular endothelial cells, and vascular smooth muscle cells also contain PAI-1, suggesting that it is an important regulator of fibrinolysis at sites of vascular injury and thrombus formation.4-7 Several nonvascular cell types express PAI-1, and PAI-1 is abundant in the extracellular matrix.8-10 Therefore, PAI-1 may regulate proteolysis within the extravascular space by inhibiting plasminogen activators and secondary target proteases, such as thrombin.11,12 Considerable information regarding the in vivo function of PAI-1 has been obtained from analyses of PAI-1–deficient mice generated by homologous recombination in embryonic stem cells.13 Vascular fibrinolysis is accelerated in PAI-1−/− mice.14 In addition, PAI-1−/− mice are resistant to the development of pulmonary fibrosis after lung injury, supporting the regulation of extravascular processes by PAI-1.15
Epidemiologic studies have identified an association between elevated plasma PAI-1 and thrombotic vascular disease in humans, including myocardial infarction and deep venous thrombosis.16-18 Conversely, a few individuals with reduced PAI-1 expression and excessive bleeding have been described.19-21 We previously reported a child with complete PAI-1 deficiency and abnormal bleeding after trauma and surgery.22 DNA sequence analysis showed that this individual was homozygous for a dinucleotide insertion within exon 4 of the PAI-1 gene. The insertion shifted the PAI-1 reading frame, resulting in premature stop codon formation and the synthesis of a truncated, nonfunctional PAI-1 protein. Therefore, this child provided a unique opportunity to study the effects of a defined abnormality of PAI-1 expression on hemostasis. The purpose of this study was to provide a more comprehensive description of the phenotype associated with PAI-1 deficiency by identifying and characterizing multiple individuals from this pedigree that carry the mutation, and by following affected individuals over a prolonged period of time.
MATERIALS AND METHODS
After obtaining informed consent, peripheral venipuncture was performed and genomic DNA was prepared from leukocytes as described previously.22 Exon 4 of the PAI-1 gene was amplified by performing the polymerase chain reaction (PCR), using primers complementary to flanking intron sequences (5′ primer: CCTGACTGCAGCCCTTTGACATACA, 3′ primer: ACATCTAGAGCATTCCCTGTGGTCTTCCTC). PCR products were alkaline denatured and applied to nylon membranes (Hybond-N; Amersham, Arlington Heights, IL) as described previously.22 Allele-specific oligonucleotide hybridization was used to identify individuals carrying the previously reported null PAI-1 allele.22 Labeling and hybridization of wild-type (ACTTACTATAGTTGAA) and mutant (ACTTACTATATAGTTGAA) probes were performed as described previously.22 Enzyme-linked immunosorbent assay (ELISA) was used to measure PAI-1 protein concentration in serum samples, using rabbit polyclonal anti–PAI-1 antibodies.22 23 Detailed bleeding histories and physical examinations were performed to determine the pattern and severity of abnormal bleeding in family members.
RESULTS
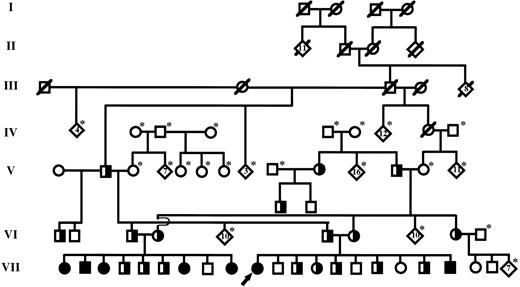
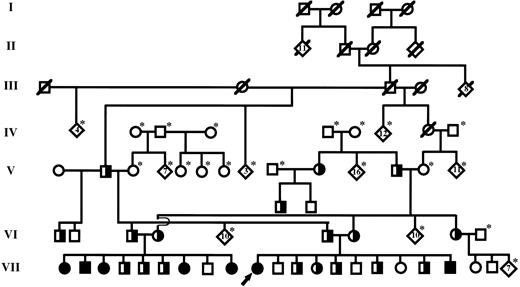
The family described in our original report was of Old Order Amish descent. Because detailed geneologic records had been maintained by family members, it was possible to track ancestors of the proband back through six generations, as shown in Fig 1. Individual III-3, who is a common ancestor to both parents of the proband, is highly likely to have carried at least one copy of the mutant PAI-1 allele. This male, born in 1881, had 17 children in two marriages and at least 142 grandchildren. He also had 8 siblings. We have confirmed by allele-specific oligonucleotide (ASO) hybridization analysis that at least one of his children by his second wife carries the mutation. Therefore, it is possible that this individual, who married into the Amish community, may represent a founder responsible for a relatively high prevalence of the mutant PAI-1 allele within this restricted population.
Simplified diagram of the extended pedigree identified by the previously reported proband (denoted by arrow).22 Individuals within each generation (I-VII) are numbered consecutively from left to right (eg, proband is individual VII-10). Squares and circles represent males and females, respectively. Partially filled symbols represent individuals that carry a single copy of the null PAI-1 allele (heterozygotes). Completely filled symbols represent individuals that carry two copies of the null allele and are completely PAI-1 deficient (homozygous affected). Individuals known to be deceased are denoted by a slash mark. Living individuals not genotyped are denoted by an asterisk. Diamonds represent the indicated number of siblings.
Simplified diagram of the extended pedigree identified by the previously reported proband (denoted by arrow).22 Individuals within each generation (I-VII) are numbered consecutively from left to right (eg, proband is individual VII-10). Squares and circles represent males and females, respectively. Partially filled symbols represent individuals that carry a single copy of the null PAI-1 allele (heterozygotes). Completely filled symbols represent individuals that carry two copies of the null allele and are completely PAI-1 deficient (homozygous affected). Individuals known to be deceased are denoted by a slash mark. Living individuals not genotyped are denoted by an asterisk. Diamonds represent the indicated number of siblings.
After establishing the diagnosis of PAI-1 deficiency in the proband, it was discovered that the brother of her father had married the sister of her mother (Fig 1). Therefore, we performed ASO hybridization analysis to determine if these individuals (ie, VI-3 and VI-4) carried the null PAI-1 allele. As shown in Fig 2, these studies confirmed that each parent carried a single copy of the exon 4 mutation. Analysis of their nine children showed that 5 were homozygous for the mutation, 3 were heterozygotes, and a single child did not carry the mutation. Serum samples were available from 4 of the children that were homozygous for the mutant allele. PAI-1 antigen was undetectable in these samples, consistent with the DNA-based analysis. Multiple episodes of abnormal bleeding were observed in the 5 homozygous affected individuals in this family (Table 1). These included bleeding into the knee and elbow joints after minor trauma, extensive subperiosteal bleeding after minor jaw trauma, delayed bleeding after surgical repair of an inguinal hernia, prolonged bleeding after tooth extraction, and frequent bruising. Bleeding times of homozygous affected children were normal, and detailed histories showed that spontaneous bleeding (eg, periumbilical bleeding after birth, epistaxis) was not observed in these individuals. One of the affected children was born prematurely secondary to placenta previa. She subsequently was diagnosed with cerebral palsy. However, no other physical or developmental abnormalities were observed in homozygous affected children. Since establishment of the diagnosis, bleeding episodes have been treated effectively with a 5- to 7-day course of oral tranexamic acid or ε-aminocaproic acid. The parents and heterozygous PAI-1–deficient children in this family had no history of excessive bleeding.
Genotype analysis of a family containing multiple members with complete PAI-1 deficiency. DNA amplified from exon 4 of each family member was analyzed by ASO hybridization analysis as described.22 This family also is shown in Fig 1. Pedigree symbols are as described in Fig 1.
Genotype analysis of a family containing multiple members with complete PAI-1 deficiency. DNA amplified from exon 4 of each family member was analyzed by ASO hybridization analysis as described.22 This family also is shown in Fig 1. Pedigree symbols are as described in Fig 1.
Three additional children have been born to the parents of the original proband since our first report.22 One child did not carry the mutation, one child was heterozygous, and a third child was homozygous for the mutant allele. The homozygous affected child, a boy, developed irritability and pallor at 4 months of age after striking his head on a table while being carried. The injury was considered trivial by the child's mother, and medical attention was not sought initially. During subsequent evaluation of persistent scalp swelling the child was found to be anemic (hemoglobin [Hgb] level, 7.8 g/dL) and to exhibit subtle motor abnormalities during neurologic examination. Head computerized tomography showed the presence of a large epidural hematoma, which required surgical drainage. ε-aminocaproic acid (10 mg/kg) was administered perioperatively, and no significant bleeding was noted after surgery. We have obtained additional clinical information on the proband, who has now been observed for 6 years since the original diagnosis of PAI-1 deficiency. Her initial management consisted of intermittent tranexamic acid, which was taken orally for 3 to 5 days after an episode of excessive bleeding or for several days before and after planned surgical procedures. This approach led to a significant improvement in her bleeding disorder. For example, after pretreatment with tranexamic acid the proband underwent extensive oral surgery and palatal biopsy without bleeding complications. However, upon initiation of menses at age 13, prolonged menstrual bleeding, typically exceeding 10 days in duration, frequently was observed. Menorrhagia did not respond adequately to tranexamic acid initiated on the first day of each menstrual cycle, and iron deficiency anemia (Hgb level, 10.0 g/dL) was noted. Tranexamic acid subsequently was taken continuously at low dose (25 mg/kg, one to two times per day) with an increase of the dose frequency to three times per day for the first few days of each menstrual cycle. On this regimen, the menorrhagia improved substantially, and the anemia resolved.
In addition to the 7 individuals with complete PAI-1 deficiency, we have identified 19 individuals that are heterozygous for the null PAI-1 mutation (see Fig 1, which shows all but one of the heterozygotes). Excessive bleeding has not been observed in any of the carriers, even after substantial trauma or surgical procedures.
DISCUSSION
In this report we have described the phenotype associated with complete PAI-1 deficiency in humans. Our analysis of this large pedigree provides convincing evidence to support the important role of PAI-1 in normal hemostasis. A spectrum of abnormal bleeding is observed in affected individuals, and although bleeding occurs only in response to trauma or surgery, it can be severe, and even life-threatening. Therefore, it appears reasonable to classify complete PAI-1 deficiency as a moderate bleeding disorder. Oral fibrinolysis inhibitors, such as tranexamic acid and ε-aminocaproic acid, are effective in preventing and treating bleeding episodes in PAI-1–deficient individuals. Heterozygous PAI-1 deficiency does not result in a clinically evident increase in bleeding, indicating that a single functional PAI-1 gene probably is sufficient for normal hemostasis. In addition to the seven individuals described in this report, three cases of abnormal bleeding due to partial PAI-1 deficiency have been reported in the medical literature. Schleef et al19 described a 76-year-old man with a lifelong history of severe bleeding after trauma or surgery, normal plasma PAI-1 antigen, but low PAI-1 activity. Dieval et al20 reported a 36-year-old man with absent plasma PAI-1 but normal platelet PAI-1 who suffered from recurrent nose bleeds and delayed bleeding after minor surgery. Lee et al21 reported a 63-year-old man with frequent nose bleed and delayed surgical bleeding, low plasma PAI-1, and normal platelet PAI-1. Although the molecular basis for partial PAI-1 deficiency was not determined in these three patients, it is unlikely that they were heterozygous for a null PAI-1 mutation, given the results of our study. It is possible that these individuals contained a mutation in the PAI-1 gene that resulted in a qualitative defect in PAI-1 function or that altered PAI-1 expression in a tissue-specific manner. Alternatively, it is possible that these individuals harbored a mutation within another factor that controls PAI-1 expression or activity, such as vitronectin.24 25
It is of interest to compare the phenotype observed in human PAI-1 deficiency with that of mice engineered to be completely deficient in PAI-1 by gene targeting (PAI-1−/− mice).13 Like humans, these animals exhibit enhanced fibrinolysis in vivo, but do not bleed spontaneously.14 Of note, PAI-1−/− mice do not bleed excessively after partial amputation of the cecum or tail, standard methods of hemostatic challenge in mice. Analysis of individuals with PAI-1 deficiency also offers important insights into the function of this protein in vivo. It appears that clinical abnormalities resulting from lack of PAI-1 expression in humans may be restricted to the blood coagulation and fibrinolytic systems, because developmental or other physical anomalies were not observed in individuals with complete lack of PAI-1 expression (except for one child born prematurely, who subsequently was diagnosed with cerebral palsy). These clinical observations are consistent with the lack of developmental abnormalities in PAI-1−/− mice as well as transgenic mice that lack other components of the plasminogen activation system, including plasminogen, t-PA, and u-PA.26-29 Because of the relatively young ages of the affected individuals identified in our study, we cannot exclude that PAI-1 may play an important role in human fertility and embryogenesis. Although our studies indicate that a fetal source of PAI-1 is not necessary for human embryonic development, it is possible that maternal PAI-1 is involved in embryo implantation and development. However, studies of PAI-1−/− mice by Carmeliet et al13 30 indicate that maternal PAI-1 is not necessary for normal embryonic development. Additional follow-up of the individuals identified in our study will be necessary to assess the potential impact of maternal PAI-1 on human fertility.
In summary, PAI-1 deficiency is an uncommon disorder that produces a moderate hemostatic defect in humans. However, it is likely that additional cases of PAI-1 deficiency will be diagnosed as awareness of this condition increases, and it is important for clinicians to consider this diagnosis in individuals with excessive bleeding, but no evidence of a hemostatic defect on standard laboratory testing. Correct diagnosis of PAI-1 deficiency is important because this disorder can be treated effectively with fibrinolysis inhibitors such as ε-aminocaproic acid and tranexamic acid, thereby possibly avoiding the use of blood products. Diagnosis of PAI-1 deficiency from plasma samples can be difficult, because PAI-1 can be undetectable in plasma from normal individuals. Therefore, we recommend measurement of PAI-1 antigen and activity in both plasma and serum as a reasonable method by which to screen individuals for possible PAI-1 deficiency.
ACKNOWLEDGMENT
We thank the individuals who participated in this study. We also thank Dr David Ginsburg (University of Michigan, Ann Arbor) for his assistance and advice while these studies were performed, and for reviewing the manuscript.
Supported by National Institutes of Health Grant No. HL-02728 to W.P.F.
Address reprint requests to Amy D. Shapiro, MD, Department of Pediatrics, James Whitcomb Riley Hospital for Children, Room 2720, 702 Barnhill Dr, Indianapolis, IN 46202-5225.