Abstract
The involvement of focal adhesion kinase (FAK) in myeloid differentiation was investigated in primary murine bone marrow (BM) cells. In unstimulated BM, FAK mRNA was detected in myeloid and lymphoid cells, but not in erythroid precursors. When the BM cells were incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF ) or interleukin-3 (IL-3), FAK expression showed a remarkable difference depending on the cytokine. Although FAK was upregulated in the cells stimulated by GM-CSF (GM-treated cells), the kinase was barely detectable in the cells cultured with IL-3 (IL-3–treated cells). Morphology and flow cytometry analysis showed GM-CSF promoted the growth and differentiation of monocyte/macrophage lineage stronger than IL-3. In addition, motility of the cytokine-differentiated cells showed an overt distinction between the cultures, which was closely correlated with FAK expression. After 7 days of stimulation, GM-treated cells showed active migration and chemoattractant-induced morphologic polarization. In contrast, IL-3–treated cells showed minimal migration and polarization. These results suggest an important role of GM-CSF in the terminal differentiation of monocytes/macrophages, and possible involvement of FAK in functional maturity of this lineage.
FOCAL ADHESION KINASE (FAK), initially identified as a unique cytoplasmic tyrosine kinase involved in focal adhesions,1-3 has been studied extensively in fibroblasts. When fibroblasts attach to the extracellular matrix and cell surface integrins recognize fibronectin or other ligands, FAK is promptly phosphorylated.4,5 Signals of activated FAK are then transmitted through Src homology 2 domain-containing adapter protein(s) (eg, Grb2), to the mitogen-activated kinase pathway, which regulates cell proliferation.6 Some neuropeptides (eg, bombesin, endothelin, and vasopressin) and growth factors (eg, platelet-derived growth factor and macrophage colony-stimulating factor) have been shown to activate FAK on binding to target cells.7-9 Moreover, in Src-transformed fibroblasts, FAK is constitutively activated and appears to be involved in maintaining the transformed phenotype.1,10 These findings suggest that FAK is located in the signal crossroads of cell growth and attachment, and involved in dynamic cytoskeletal rearrangements.11
The significance of FAK in the lympho-hematopoietic system has been studied in platelet aggregation,12-14 in signal transduction in T lymphocytes,15-17 and in Ig E receptor aggregation.18-20 Less is known about its involvement in myeloid and erythroid development, where its role is somewhat controversial. It was reported that the expression of FAK in granulocyte-macrophage (GM) lineage and mast cells was minimal,21 but in other reports, FAK expression has been shown in human peripheral monocytes9 as well as in a rat basophilic leukemia line.18-20 We began the present study by examining which hematopoietic lineage was actually expressing FAK, to elucidate the possible functions of this kinase in hematopoiesis.
MATERIALS AND METHODS
Cells and culture.Bone marrow (BM) cells were harvested from C57BL/6 mice of 8 weeks of age (purchased from SLC, Hamamatsu, Japan) and cultured in α-minimum essential medium (pH 7.4) with 10% fetal bovine serum (FBS) unless otherwise stated. After depletion of the plastic-adherent cells at 37°C for 3 hours, the BM cells were incubated in the presence of either recombinant murine GM-colony-stimulating factor (GM-CSF ) (100 U/mL), or recombinant murine interleukin-3 (IL-3) (100 U/mL). Purified recombinant murine GM-CSF and IL-3 were kindly provided by Dr T. Sudo (TORAY, Kamakura, Japan).
Flow cytometry (FCM) analysis of murine BM cells.Several lineage-specific monoclonal antibodies (MoAbs) were used to separate BM cells with the aid of a FACS Vantage cell sorter (Becton Dickinson, San Jose, CA). Anti-Gr-1, anti-Mac-1, anti-TER-119, anti-B220, CD4, and CD8 Abs were purchased from Pharmingen (San Diego, CA). An anti-c-Kit MoAb (ACK-222 ) was provided by Dr S. Nishikawa (Kyoto University, Japan). To characterize cultured BM cells, fluorescein isothiocyanate (FITC)-conjugated MoAbs, specific to the monocyte/macrophage lineage (F4/80 from Serotec, Oxford, UK, and anti-c-Fms MoAb from Dr S. Nishikawa, University of Kyoto23 ) were used along with a FACScan (Becton Dickinson).
RNA preparation and reverse transcriptase-directed polymerase chain reaction (RT-PCR).Total RNA was prepared following a modified single-step isolation protocol24 with ISOGEN reagent (Nippon Gene, Toyama, Japan). The first strand cDNA was then synthesized with Superscript II (Life Technologies, Gaithersburg, MD). Typically, total RNA was extracted from 5 × 104 lineage-sorted cells and subjected to cDNA synthesis, or 2 μg of total RNA from cytokine-treated cells was subjected to cDNA synthesis. RT-PCR for FAK mRNA detection25 was performed using the upstream sequence: 5′-AGAGAATCCAGCTTTGGCTG-3′ and the downstream sequence: 5′-CAGCACTCGCGTATCTGGAG-3′.21 The oligonucleotides that were used for detection of the β-actin message were as follows: upstream sequence, 5′-TCGTGCGTGACATCAAAGAG-3′ and downstream sequence, 5′-CATCCTGGCCTCACTGTCCA-3′.26
Immunochemical analysis.BM-derived cells were lysed in Triton lysis buffer (50 mmol/L HEPES [pH 7.4], 1% Triton X-100 (Sigma, St Louis, MO), 4 mmol/L EDTA, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, 1 mmol/L phenylmethylsulfonyl fluoride). Protein was quantitated by BCA Protein Assay (Pierce, Rockford, IL) and 20 μg of each sample was separated on a polyacrylamide gel.27 After transfer onto Immobilon-P polyvinylidene difluoride (PVDF ) membrane (Nihon Millipore, Yonezawa, Japan),28 the blots were hybridized with an antimouse FAK rabbit Ab (JF1; a generous gift from Dr J. Fujimoto, University of Tokyo, Japan)16 29 and the FAK protein was visualized with an enhanced chemiluminescence system (Amersham, Little Chalfont, UK). The blots were then reprobed with an anti-Mac-1 MoAb (Pharmingen). In some experiments, lysates were immunoprecipitated with an anti-FAK MoAb (2A7; Upstate Biotechnology, Lake Placid, NY) for probing with JF1 antibody.
Motility assays.Random migration of murine BM-derived cells was assayed in a modified Boyden chamber (Neuro Probe, Bethesda, MD) with a Nuclepore polycarbonate filter with micropores of 5 μm (Costar, Bedford, MA).30 After 7 days of culture with either GM-CSF or IL-3, nonadherent cells were harvested, extensively washed with phosphate-buffered saline (PBS, pH 7.4), and resuspended in RPMI with 10% FBS at 1 × 106 cells/mL. The lower well of the chamber was filled with PBS, and a 50 μL aliquot of the cell suspension placed in the upper well. After incubation at 37°C for 90 minutes, the filter was Giemsa stained and migration to the underside measured. For each chamber, the number of migrated cells in four randomly chosen high power fields (HPF; original magnification × 400) was counted.
Morphologic polarization assay was performed according to the method of Cianciolo and Snyderman,31 to evaluate the responsiveness of cultured GM cells to chemoattractants such as N-formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma) and human complement C5a (Sigma). The cytokine-treated cells were prepared as in random migration assay and resuspended at 1 × 106 cells/mL in Hanks' balanced salt solution (pH 7.4) with 0.5% bovine serum albumin. A 270 μL aliquot of cell suspension was mixed with 30 μL of 10−8 mol/L fMLP (final concentration 10−9 mol/L) or 10−7 mol/L C5a (final concentration 10−8 mol/L) and incubated at 37°C for 10 minutes. The cells were then fixed by adding 1 mL of 8% paraformaldehyde and stained with staining solution (0.1% Fast Blue BB Salt [Sigma] and 0.024% α-naphthyl acetate [Sigma] in PBS). Cells with characteristic morphologic alteration were determined as “polarized” by microscopic examination, and the polarization activity of cells was calculated by the following formula: Polarization activity = (morphologically polarized cells/total cells) × 100(%).
RESULTS
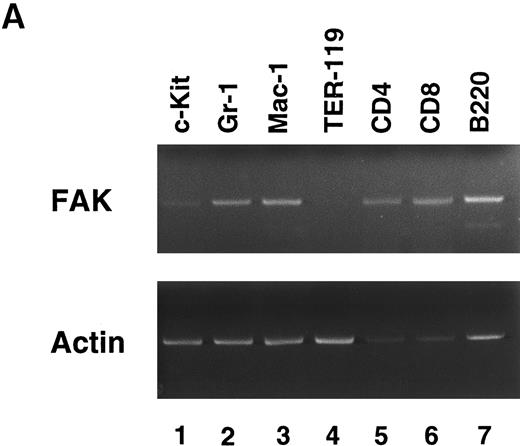
FAK expression in hematopoietic cells.To determine which hematopoietic lineage expresses FAK, we separated murine BM cells by sorting with lineage-specific MoAbs. Figure 1A shows the results of an RT-PCR of lineage-sorted cells. FAK was weakly expressed in c-Kit+ cells (lane 1), and readily detected in GM (Gr-1 and Mac-1; lanes 2 and 3) and lymphoid (CD4, CD8, and B220; lanes 5 through 7) cells. However, FAK mRNA was barely detected in erythroid precursors (TER-119; lane 4). Repeated sorting and RT-PCR failed to detect FAK expression in TER-119+ cells. Thus, FAK was expressed in all the hematopoietic lineages with the exception of erythroid cells.
RT-PCR analysis of lineage-sorted (A) and cytokine-treated (B) murine BM cells. (A) Bone marrow cells from C57BL/6 mice were sorted for RT-PCR to detect FAK mRNA (upper panel) and β-actin mRNA (lower panel). The MoAbs used in sorting were anti-c-Kit (lane 1), Gr-1 (lane 2), Mac-1 (lane 3), TER-119 (lane 4), CD4 (lane 5), CD8 (lane 6), and B220 (lane 7). (B) Day 7 GM-treated cells (lane 3, adherent cells; lane 4, nonadherent cells) and IL-3–treated cells (lane 5, adherent cells; lane 6, nonadherent cells) were subjected to RT-PCR to detect FAK mRNA (upper panel) and β-actin mRNA (lower panel). Unstimulated controls were also analyzed (lane 1, adherent cells; lane 2, nonadherent cells).
RT-PCR analysis of lineage-sorted (A) and cytokine-treated (B) murine BM cells. (A) Bone marrow cells from C57BL/6 mice were sorted for RT-PCR to detect FAK mRNA (upper panel) and β-actin mRNA (lower panel). The MoAbs used in sorting were anti-c-Kit (lane 1), Gr-1 (lane 2), Mac-1 (lane 3), TER-119 (lane 4), CD4 (lane 5), CD8 (lane 6), and B220 (lane 7). (B) Day 7 GM-treated cells (lane 3, adherent cells; lane 4, nonadherent cells) and IL-3–treated cells (lane 5, adherent cells; lane 6, nonadherent cells) were subjected to RT-PCR to detect FAK mRNA (upper panel) and β-actin mRNA (lower panel). Unstimulated controls were also analyzed (lane 1, adherent cells; lane 2, nonadherent cells).
FAK expression in cultured BM cells.RT-PCR analysis suggested that FAK was expressed weakly in primitive hematopoietic cells, then upregulated along with myeloid and lymphoid differentiation. Next, we investigated the effects of GM-CSF and IL-3 on murine myeloid growth and differentiation in vitro. Figure 2 shows the growth curves of cultured BM cells. Starting with 1 × 107 cells devoid of plastic-adherent cells, culture with GM-CSF (GM-CSF culture) yielded 5.1 ± 1.4 × 106 adherent cells and 8.8 ± 1.4 × 106 nonadherent cells on day 7, whereas culture with IL-3 (IL-3 culture) yielded 2.5 ± 0.8 × 106 adherent cells and 9.3 ± 4.4 × 106 nonadherent cells on the same day (mean ±SD; n = 3). Thus, the GM-CSF stimulation resulted in twice as many adherent cells as IL-3 during 1 week, and both cultures yielded comparable numbers of nonadherent cells, which peaked on day 5 (Fig 2).
Growth curves of cytokine treated bone marrow. Each culture was started with 1 × 107 cells after depletion of plastic-adherent cells and incubated with either 100 U/mL GM-CSF or 100 U/mL IL-3. Number of the recovered cells of each time point represents mean ±SD (n = 3). •, adherent cells in GM-CSF culture; ▪, nonadherent cells in GM-CSF culture; ○, adherent cells in IL-3 culture; □, nonadherent cells in IL-3 culture.
Growth curves of cytokine treated bone marrow. Each culture was started with 1 × 107 cells after depletion of plastic-adherent cells and incubated with either 100 U/mL GM-CSF or 100 U/mL IL-3. Number of the recovered cells of each time point represents mean ±SD (n = 3). •, adherent cells in GM-CSF culture; ▪, nonadherent cells in GM-CSF culture; ○, adherent cells in IL-3 culture; □, nonadherent cells in IL-3 culture.
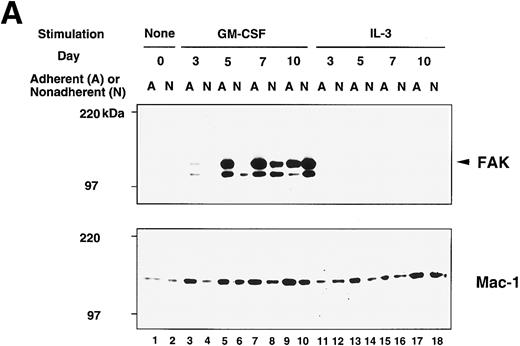
Although the growth stimulation effect was within twofold range, the occurrence of FAK protein showed a striking difference between GM-treated cells and IL-3–treated cells, which is illustrated in the upper panel of Fig 3A. FAK protein was not detected in unstimulated cells (day 0; lanes 1 and 2) but appeared on day 5 in adherent population of GM-CSF culture (lane 5). Expression was increased in the adherent cells and reached a plateau on day 7 (lane 7). FAK was also detected on day 7 and day 10 nonadherent cells of GM-CSF culture (lanes 8 and 10). In contrast, a very small amount, if any, of FAK protein was detected in IL-3–treated cells (lanes 11 through 18). When IL-3 was added to the culture together with GM-CSF, the amount of FAK protein was slightly less than that of cells cultured only with GM-CSF (not shown). In this immunoblot analysis, the anti-FAK Ab (JF1) detected 125-kD (arrowhead in Fig 3) and 110-kD peptides in BM lysates, while the same Ab detected only the 125-kD peptide in NIH3T3 fibroblast lysate (not shown). In addition, when lysate of GM-treated cells was immunoprecipitated with an anti-FAK MoAb (2A7) and probed with JF1, only 125-kD species was detected (not shown). Thus, it is likely that the 110-kD peptide was one of the FAK-related kinases (PYK2, CAKβ, RAFTK),32-34 which might have cross-reacted with JF1 Ab.
Immunoblot analysis of murine BM culture after GM-CSF or IL-3 stimulation. (A) The BM cells were incubated in the presence of GM-CSF or IL-3 for 3, 5, 7, and 10 days. Cell lysates (20 μg/lane) were analyzed by immunoblotting with anti-murine FAK rabbit antibody JF1 (upper panel) and reprobed with anti-Mac-1 Ab (lower panel). Lanes 1 and 2, unstimulated BM; lanes 3 through 10, stimulated by GM-CSF; lanes 11 through 18, stimulated by IL-3. Lanes 1, 3, 5, 7, 9, 11, 13, 15, and 17, adherent cells; lanes 2, 4, 6, 8, 10, 12, 14, 16, and 18, nonadherent cells. Arrowhead indicates FAK protein (125 kD). (B) Immunoblot analysis of murine BM before and after cytokine switching. The BM cells were incubated with GM-CSF or IL-3 for 5 days, before switching to the other growth factor for another 5 days. Cell lysates (20 μg/lane) were immunoblotted with JF1 anti-FAK Ab (upper panel) and reprobed with anti-Mac-1 Ab (lower panel). Lanes 1 and 2, unstimulated BM; lanes 3 and 4, day 5 cell lysates after stimulation with IL-3 only; lanes 5 and 6, cytokine switching from IL-3 to GM-CSF; lanes 7 and 8, day 5 cell lysates after stimulation with GM-CSF only; lanes 9 and 10, cytokine switching from GM-CSF to IL-3. Lanes 1, 3, 5, 7, and 9, adherent cells; lanes 2, 4, 6, 8, and 10, nonadherent cells. Arrowhead indicates FAK protein (125 kD).
Immunoblot analysis of murine BM culture after GM-CSF or IL-3 stimulation. (A) The BM cells were incubated in the presence of GM-CSF or IL-3 for 3, 5, 7, and 10 days. Cell lysates (20 μg/lane) were analyzed by immunoblotting with anti-murine FAK rabbit antibody JF1 (upper panel) and reprobed with anti-Mac-1 Ab (lower panel). Lanes 1 and 2, unstimulated BM; lanes 3 through 10, stimulated by GM-CSF; lanes 11 through 18, stimulated by IL-3. Lanes 1, 3, 5, 7, 9, 11, 13, 15, and 17, adherent cells; lanes 2, 4, 6, 8, 10, 12, 14, 16, and 18, nonadherent cells. Arrowhead indicates FAK protein (125 kD). (B) Immunoblot analysis of murine BM before and after cytokine switching. The BM cells were incubated with GM-CSF or IL-3 for 5 days, before switching to the other growth factor for another 5 days. Cell lysates (20 μg/lane) were immunoblotted with JF1 anti-FAK Ab (upper panel) and reprobed with anti-Mac-1 Ab (lower panel). Lanes 1 and 2, unstimulated BM; lanes 3 and 4, day 5 cell lysates after stimulation with IL-3 only; lanes 5 and 6, cytokine switching from IL-3 to GM-CSF; lanes 7 and 8, day 5 cell lysates after stimulation with GM-CSF only; lanes 9 and 10, cytokine switching from GM-CSF to IL-3. Lanes 1, 3, 5, 7, and 9, adherent cells; lanes 2, 4, 6, 8, and 10, nonadherent cells. Arrowhead indicates FAK protein (125 kD).
By densitometric comparison with NIH3T3 lysate, we estimated that cultured BM cells had only 1% to 5% of FAK protein on day 7 of GM-CSF stimulation (not shown). Because of the low abundance and probably dispersed distribution of FAK protein in the GM-treated cells, we were not able to localize FAK by immunofluorescent microscopy. Therefore, one might argue that contaminated other cell types in the adherent cells, eg, fibroblasts and stromal cells, could account for the major source of the kinase. However, this is not likely the case, because we started the cultures after depletion of plastic-adherent cells, and the unstimulated cells did not express FAK (Fig 3A, lanes 1 and 2; Fig 3B, lanes 1 and 2). In addition, all the recovered cells were myeloid cells after 7 days of GM-CSF stimulation (see below and Fig 4A). Therefore, we consider the FAK protein detected in the immunoblot is myeloid origin but not from other cell types.
(A) Differential counts of GM-treated cells and IL-3–treated cells. Adherent and nonadherent cells were recovered on day 7 and the cytospin slides were subjected to May-Grünwald-Giemsa staining. (B) FCM analysis of murine BM cells after GM-CSF or IL-3 stimulation. The nonadherent BM-derived cells were analyzed for F4/80 expression (left panels) and c-Fms expression (right panels). Cells were harvested on day 0 (top panels), and on day 7 of either GM-CSF (middle panels) or IL-3 (bottom panels) stimulation. Solid line, stained with F4/80-FITC or anti-c-Fms-FITC; dotted line, unstained.
(A) Differential counts of GM-treated cells and IL-3–treated cells. Adherent and nonadherent cells were recovered on day 7 and the cytospin slides were subjected to May-Grünwald-Giemsa staining. (B) FCM analysis of murine BM cells after GM-CSF or IL-3 stimulation. The nonadherent BM-derived cells were analyzed for F4/80 expression (left panels) and c-Fms expression (right panels). Cells were harvested on day 0 (top panels), and on day 7 of either GM-CSF (middle panels) or IL-3 (bottom panels) stimulation. Solid line, stained with F4/80-FITC or anti-c-Fms-FITC; dotted line, unstained.
To further investigate the effects of GM-CSF and IL-3 on murine BM, we exchanged the growth factors during in vitro culture and analyzed FAK expression. After 5 days of incubation with GM-CSF or IL-3, the cells were washed and allowed to grow for another 5 days in the presence of the other growth factor. Figure 3B shows the results of cytokine switching. Clearly, the subsequent GM-CSF stimulation on the IL-3–treated cells promoted FAK expression strongly in the adherent cells and weakly in the nonadherent cells (Fig 3B, upper panel, lanes 3 through 6). On the other hand, FAK expression in BM cells decreased after switching from GM-CSF to IL-3 (Fig 3B, upper panel, lanes 7 through 10).
When the FAK blots were reprobed with an anti-Mac-1 Ab (Fig 3A and 3B, lower panels), Mac-1 was shown to be distributed evenly in the cells whether they were incubated with GM-CSF or IL-3, showing that both growth factors strongly stimulated myeloid cells in culture.
FAK mRNA detection in cytokine-treated cells.Since we observed an apparent difference of FAK protein between GM-treated cells and IL-3–treated cells, we next investigated whether FAK was induced at transcriptional level by GM-CSF. Conventional Northern blot analysis did not detect significant FAK mRNA in cultured myeloid cells, whether 20 μg of total RNA or 2 μg of poly(A)+RNA was analyzed. Thus, we performed RT-PCR analysis on GM-treated cells and IL-3–treated cells to detect FAK mRNA (Fig 1B). The RT-PCR study showed minimal FAK mRNA in unstimulated cells, and almost evenly increased levels of FAK message in GM-treated and IL-3–treated adherent cells. FAK mRNA was detected in the nonadherent cells after GM-CSF or IL-3 stimulation, but the extents of the increase were smaller than in the adherent cells. These results suggest that FAK upregulation in cytokine-treated myeloid cells is controlled at posttranscriptional level, such as translational efficiency or stability of the protein in the cell.
Morphology and surface markers of cultured BM cells.Because the GM-treated cells and IL-3–treated cells showed a striking difference in FAK expression, we examined other phenotypes of those cells such as morphology and expression of myeloid differentiation markers. May-Grünwald-Giemsa staining of the cytospin slides showed strong GM differentiation after incubation with either growth factor, with more preferential growth and maturation of monocytes/macrophages after GM-CSF stimulation. Figure 4A shows the differential counts of the cytokine-treated cultures. GM-treated adherent cells contained 70% monocytes/macrophages and 30% granulocytes; GM-treated nonadherent cells contained 55% monocytes/macrophages, 40% granulocytes, and 5% blasts; IL-3–treated adherent cells contained 50% monocytes/macrophages, 40% granulocytes, and 10% myelocytes; IL-3–treated nonadherent cells contained 30% monocytes/macrophages, 40% granulocytes, 25% blasts, and 5% lymphocytes. When incubated with Latex beads, cells with monocyte/macrophage morphology showed active phagocytosis, regardless of whether they had been stimulated by GM-CSF or IL-3 (not shown).
FCM analysis of leukocyte integrins such as Mac-1, VLA-4, and VLA-5, showed an almost even distribution in both GM-treated cells and IL-3–treated cells (not shown). When monocyte/macrophage lineage-specific markers such as F4/80 and c-Fms were examined, GM-CSF promoted the expression of those markers in nonadherent cells stronger than IL-3 did (Fig 4B). These results also imply that GM-CSF promoted more monocytic differentiation and maturation than IL-3.
Motility of cultured myeloid cells.When GM-treated cells and IL-3–treated cells were assayed for their motility, an apparent difference was observed (Fig 5). In random migration assay, only background number of IL-3–treated cells migrated across a multiporous membrane. In contrast, numerous cells from the GM-CSF culture migrated to the underside, showing active motility (Fig 5A). This difference was quite reproducible in repeated experiments, with 40- to 65-fold more cells migrating after GM-CSF stimulation than after IL-3 stimulation. To exclude the possibility that this difference was merely due to the chemotactic activity of GM-CSF itself, we costimulated the IL-3–treated cells with GM-CSF for up to 16 hours before assay, but this procedure did not increase the motility of IL-3–treated cells at all (time course not shown).
Motility assays of cytokine-treated cells. (A) Random migration assay across a multiporous filter. Nonadherent cells were recovered on day 7 of GM-CSF (middle) or IL-3 (right) treatment and placed in the upper wells of a modified Boyden chamber in triplicate. After 90 minutes of incubation at 37°C, the filter was Giemsa stained and the migrated cells to the underside were counted in four randomly chosen HPFs (original magnification × 400). Bars represent the numbers of migrated cells per HPF (mean ± SD). Control, unstimulated cells. (B) Morphologic polarization activity of myeloid cells. Day 7 GM-treated and IL-3–treated nonadherent cells were either preincubated with GM-CSF for 30 minutes before assay (priming [+]) or left unprimed until assay (priming [−]). Cells were challenged by PBS, fMLP, or C5a for 10 minutes to evoke polarization. Bars show the polarization activities of cells calculated as in Materials and Methods, after being challenged by PBS (□), fMLP (), or C5a (▪). (C and D) Polarized cells on C5a stimulation. More than 50% of the GM-treated cells (priming [+]) underwent characteristic morphological changes (C; original magnification × 150), while the IL-3–treated cells contained much fewer polarized cells with milder alterations in shape, even with priming (D; original magnification × 150).
Motility assays of cytokine-treated cells. (A) Random migration assay across a multiporous filter. Nonadherent cells were recovered on day 7 of GM-CSF (middle) or IL-3 (right) treatment and placed in the upper wells of a modified Boyden chamber in triplicate. After 90 minutes of incubation at 37°C, the filter was Giemsa stained and the migrated cells to the underside were counted in four randomly chosen HPFs (original magnification × 400). Bars represent the numbers of migrated cells per HPF (mean ± SD). Control, unstimulated cells. (B) Morphologic polarization activity of myeloid cells. Day 7 GM-treated and IL-3–treated nonadherent cells were either preincubated with GM-CSF for 30 minutes before assay (priming [+]) or left unprimed until assay (priming [−]). Cells were challenged by PBS, fMLP, or C5a for 10 minutes to evoke polarization. Bars show the polarization activities of cells calculated as in Materials and Methods, after being challenged by PBS (□), fMLP (), or C5a (▪). (C and D) Polarized cells on C5a stimulation. More than 50% of the GM-treated cells (priming [+]) underwent characteristic morphological changes (C; original magnification × 150), while the IL-3–treated cells contained much fewer polarized cells with milder alterations in shape, even with priming (D; original magnification × 150).
This contrast in random migration between GM-treated cells and IL-3–treated cells was paralleled in the morphologic polarization assay, in which day 7 GM-treated and IL-3–treated nonadherent cells were evaluated for their responsiveness to chemoattractants such as fMLP and C5a (Fig 5B through D). In this experiment, GM-CSF was also superimposed onto aliquots of GM-CSF and IL-3 cultures before assay, to monitor the “priming” effect that GM-CSF may have on cellular responsiveness to subsequent stimuli.35 A moderate priming effect was observed in GM-treated cells, which peaked at 30 minutes, but no priming effect was observed in IL-3–treated cells (time course not shown). As seen in Fig 5B, GM-treated cells had enhanced basal polarization activity (25% to 30%) compared with IL-3–treated cells (<5%). Thirty minutes of “priming” further increased the responsiveness of GM-treated cells to the second stimulus by fMLP or C5a (50% to 55%), while IL-3–treated cells stayed inert even after priming. The extents of polarization of GM-treated cells were remarkable (Fig 5C), while IL-3–treated cells showed only mild polarization at much lower frequency (Fig 5D). Thus, the enhanced polarization and migration of GM-treated cells may be attributed to the relatively long-term effect of GM-CSF, while the inertness of IL-3–treated cells suggests that they lacked locomotive machinery.
DISCUSSION
In the present study, we evaluated FAK expression in murine hematopoietic cells and showed that FAK was expressed in the myeloid and lymphoid cells. This tyrosine kinase was expressed in c-Kit positive primitive cells in small amounts, but barely expressed in erythroid precursors. FAK expression in GM lineage was consistent with the previous findings that it was expressed in monocytes and a basophilic leukemia line.9,18-20 For erythroid lineage, however, our RT-PCR study has a discrepancy from a previous report in which Choi et al21 showed FAK mRNA in erythroid precursors. They analyzed embryonic or cultured cells including transformed cell lines, whereas we analyzed fresh BM cells from adult mice. This difference of RNA source may account for the discrepancy between studies, but this is yet to be clarified.
When FAK expression was studied at protein level in the cultured bone marrow cells stimulated by GM-CSF, upregulation of this kinase was substantiated in these cells. Unexpectedly, IL-3 was unable to promote FAK expression in cultured BM cells, even though it is well documented that both cytokines strongly support proliferation and differentiation of GM progenitor cells. The underlying mechanisms of the overt contrast between GM-CSF and IL-3 in FAK-upregulating ability is of particular interest. Our RT-PCR study showed FAK mRNA in both GM-treated cells and IL-3–treated cells, which suggests that FAK upregulation may be controlled at posttranscriptional level. Presumably some additional factors are required for efficient FAK translation or stabilizing it in the differentiated myeloid cells. There are several possibilities that could account for a difference observed in our study. (1) GM-CSF and IL-3 work in different ways in the same cell. That is, GM-CSF upregulates FAK in late stages of myeloid differentiation, but IL-3 does not. (2) FAK upregulation in myeloid cells is subpopulation-specific. That is, some subpopulation of cells (eg, c-Fms+ cells) preferentially respond to GM-CSF stimulation and express FAK, but IL-3 does not support such cells strongly. (3) FAK upregulation is an indirect result of GM-CSF/IL-3 treatment on heterogeneous population, ie, other cell types may produce some factor(s) to promote or inhibit FAK expression in myeloid cells.
Among the possibilities mentioned above, we are particularly interested in the first mechanism. IL-3, IL-5, and GM-CSF receptors share a common β chain and presumably share common signaling pathways.36,37 It has not been clarified whether or not the same GM progenitors coexpress GM-CSF and IL-3 receptors and yet respond differently. If this is actually the case, there would be α-chain–specific signal transduction or modification pathways in the cytokine receptor family (IL-3, IL-5, and GM-CSF receptors). Takaki et al38 showed the importance of the cytoplasmic portion of murine IL-5 receptor α-chain in a transfected cell line, and Mire-Sluis et al39 suggested a signaling role for the α-chains of the receptor family in a human erythroleukemia line. Analysis of FAK upregulation by cytokines would be facilitated by developing an experimental system, in which one can activate GM-CSF receptor in more defined cell lines.
While GM-CSF and IL-3 have redundant roles in hematopoiesis, it has been postulated that IL-3 may principally support myeloid progenitors and GM-CSF exerts its effect through more differentiated macrophages. Our FCM study seems to support this notion and FAK upregulation in GM-treated cells may represent another aspect of macrophage differentiation. In this regard, more detailed study on subpopulation-separated cells for their responsiveness to GM-CSF or IL-3 is awaited.
When assayed for motility, GM-treated cells and IL-3–treated cells showed an overt distinction that the former population showed active migration and polarization while the latter stayed inert. Although the contents of monocytes/macrophages and granulocytes were not identical in the assayed populations, we do not consider this factor greatly affected the results of motility assays for the following reasons. (1) Cells were assayed immediately after extensive washing so that the culture media would least influence the motility. (2) Motility distinction was confirmed by two different assays, and the cells were incubated for only 10 minutes in polarization assay. Within this short period, it is unlikely that one cell type in the mixture produces enough amount of additional stimulators or inhibitors of migration. (3) Even with the prestimulation or priming with GM-CSF, the nonadherent cells from IL-3 culture showed practically no motility. Therefore, the results of our motility assays suggest the presence of a subset of cells capable of migrating in GM-treated nonadherent cells, and absence of such a subset in IL-3–treated counterpart.
Several studies have revealed that FAK is involved in cell motility and deformity by controlling cytoskeletal rearrangement. Growth factors such as platelet-derived growth factor and hepatocyte growth factor induced phosphorylation in aortic vascular smooth muscle cells and oral squamous cell carcinoma, respectively, and concomitantly promoted migration of these cells.8,40 Stimulation by hyaluronan, an extracellular matrix glycosaminoglycan, resulted in the rapid phosphorylation of FAK and increased the motility of a H-Ras-transformed cell line.41 Finally, FAK-deficient mice had generalized defects of mesoderm development and died in utero, while cells from the mutant embryos showed reduced motility.29,42 For terminally differentiated macrophages, acquiring locomotive and deforming ability is critical to function. Taken together with these findings, the restrictive coexistence of FAK and motility showed herein implicates the involvement of FAK in monocyte/macrophage migration. A more supportive evidence for FAK involvement in phagocyte motility will be obtained, if one can specifically inactivate FAK in myeloid cells. FAK-deficient embryonic stem cells may provide another possible means of analysis, since they can be differentiated into macrophages by a recently developed in vitro system using OP9 stromal cells.43 Understanding the intracellular events of GM-CSF–mediated differentiation will help clarify the mechanisms of FAK upregulation in GM cells, and further studies of this multifunctional kinase should provide insight into the physiology of macrophages.
ACKNOWLEDGMENT
We are grateful to Dr M. Hashiyama for aid in FCM analysis and cell sorting. Special thanks to Dr D. Ilic and Dr S. Kanazawa for their thoughtful comments.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Akihiro Kume, MD, PhD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, 2-2-1 Honjo, Kumamoto 860, Japan.






![Fig. 5. Motility assays of cytokine-treated cells. (A) Random migration assay across a multiporous filter. Nonadherent cells were recovered on day 7 of GM-CSF (middle) or IL-3 (right) treatment and placed in the upper wells of a modified Boyden chamber in triplicate. After 90 minutes of incubation at 37°C, the filter was Giemsa stained and the migrated cells to the underside were counted in four randomly chosen HPFs (original magnification × 400). Bars represent the numbers of migrated cells per HPF (mean ± SD). Control, unstimulated cells. (B) Morphologic polarization activity of myeloid cells. Day 7 GM-treated and IL-3–treated nonadherent cells were either preincubated with GM-CSF for 30 minutes before assay (priming [+]) or left unprimed until assay (priming [−]). Cells were challenged by PBS, fMLP, or C5a for 10 minutes to evoke polarization. Bars show the polarization activities of cells calculated as in Materials and Methods, after being challenged by PBS (□), fMLP (), or C5a (▪). (C and D) Polarized cells on C5a stimulation. More than 50% of the GM-treated cells (priming [+]) underwent characteristic morphological changes (C; original magnification × 150), while the IL-3–treated cells contained much fewer polarized cells with milder alterations in shape, even with priming (D; original magnification × 150).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3434/3/m_bl_0050f5a.jpeg?Expires=1767913011&Signature=3ub5wXVTcOCLvL2wq8sOSnrqgjHHX99RgkvvOfpf2QKQ4R1AIYf5tsgKdPwAjRHbv55zIjOJASagyUHxiAzDu5inasxaZVUn6k~1vId0J4XkdNM6LY4dYayRRjKRKzDgrNa9sBcjxLa3WvkljFBP8autxc8cDnj8rxLMLLOT30nZl7o0tsNkdX4pCyvH9zMqfKZcOShU5NNFdmQlQJkAJjRAOR6z3vgQETW6k18Nn~gn5FNBKUWRXmwEbelF4fQSwQ8nNCukpIANP0VZ9ifZaV4SZaPLko7UDS06TdbUui35jxtAn4p8ZjNVDnmelnwruxjn~67sRCEiDbSuO1FTqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Motility assays of cytokine-treated cells. (A) Random migration assay across a multiporous filter. Nonadherent cells were recovered on day 7 of GM-CSF (middle) or IL-3 (right) treatment and placed in the upper wells of a modified Boyden chamber in triplicate. After 90 minutes of incubation at 37°C, the filter was Giemsa stained and the migrated cells to the underside were counted in four randomly chosen HPFs (original magnification × 400). Bars represent the numbers of migrated cells per HPF (mean ± SD). Control, unstimulated cells. (B) Morphologic polarization activity of myeloid cells. Day 7 GM-treated and IL-3–treated nonadherent cells were either preincubated with GM-CSF for 30 minutes before assay (priming [+]) or left unprimed until assay (priming [−]). Cells were challenged by PBS, fMLP, or C5a for 10 minutes to evoke polarization. Bars show the polarization activities of cells calculated as in Materials and Methods, after being challenged by PBS (□), fMLP (), or C5a (▪). (C and D) Polarized cells on C5a stimulation. More than 50% of the GM-treated cells (priming [+]) underwent characteristic morphological changes (C; original magnification × 150), while the IL-3–treated cells contained much fewer polarized cells with milder alterations in shape, even with priming (D; original magnification × 150).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3434/3/m_bl_0050f5b.jpeg?Expires=1767913011&Signature=TMTXFf3VbDEDInvHY3MQC34PjipOfbLy-WJereoEhnXTZKJ3dxUmh~OLfatG6zx0CFLfZVU~sS1f2Uiwm1k~YAgrVGyrsZFdWzmiUdzmPY6alEfw~LU9MgArkbxjxhWC9fLfxm98IQdKPPbYSA24VXdFF-MMzvmjBnHwcvaGaYNlptRzxB462xC6AFjE8EL8TlDbE1u-mcL8egLfDRwfzfig2JP8Unqr0aP8e4L7dL44TvKczg8jT1uyeRfiDEcVgSRrwFWs9ozUURA8iHa7Jv-EWTR6Qj9OPKbrDXIhnpoVC-APXWai1wi~6Edrm~frUjz5zHYPSiuQXH8rJO3QqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Motility assays of cytokine-treated cells. (A) Random migration assay across a multiporous filter. Nonadherent cells were recovered on day 7 of GM-CSF (middle) or IL-3 (right) treatment and placed in the upper wells of a modified Boyden chamber in triplicate. After 90 minutes of incubation at 37°C, the filter was Giemsa stained and the migrated cells to the underside were counted in four randomly chosen HPFs (original magnification × 400). Bars represent the numbers of migrated cells per HPF (mean ± SD). Control, unstimulated cells. (B) Morphologic polarization activity of myeloid cells. Day 7 GM-treated and IL-3–treated nonadherent cells were either preincubated with GM-CSF for 30 minutes before assay (priming [+]) or left unprimed until assay (priming [−]). Cells were challenged by PBS, fMLP, or C5a for 10 minutes to evoke polarization. Bars show the polarization activities of cells calculated as in Materials and Methods, after being challenged by PBS (□), fMLP (), or C5a (▪). (C and D) Polarized cells on C5a stimulation. More than 50% of the GM-treated cells (priming [+]) underwent characteristic morphological changes (C; original magnification × 150), while the IL-3–treated cells contained much fewer polarized cells with milder alterations in shape, even with priming (D; original magnification × 150).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3434/3/m_bl_0050f5c.jpeg?Expires=1767913011&Signature=Zyd97shDzE92P0q4~tPf26DyMG36KIRI3bLyO1afjoex7Px1t3Cl4nmFUXK~zHxj1RXdgXn7kJ6cap9aecBzwO27hDIT-DY8sCTjtwhmZA8D0vgbfjiFeqDFtLe69LzXDQuoB1ra0WcCLIJzfUEceQtAWrwiAkHygO8W9323sSffanNbmA~foUekukZAM2~I8-Na7dQ4R0Avn8AGZj-3~JotV-WHAgykb02-dmH5TNAVAoLeLFdUilcUvofNREcmcKADnuDR2W-6RuxvwzE9t~vJOHiDyX642NKrpANDqPar0VR1ZabohF8PSjZsBsyJ1NYfdTa~sqSbDfnyZJFVgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Motility assays of cytokine-treated cells. (A) Random migration assay across a multiporous filter. Nonadherent cells were recovered on day 7 of GM-CSF (middle) or IL-3 (right) treatment and placed in the upper wells of a modified Boyden chamber in triplicate. After 90 minutes of incubation at 37°C, the filter was Giemsa stained and the migrated cells to the underside were counted in four randomly chosen HPFs (original magnification × 400). Bars represent the numbers of migrated cells per HPF (mean ± SD). Control, unstimulated cells. (B) Morphologic polarization activity of myeloid cells. Day 7 GM-treated and IL-3–treated nonadherent cells were either preincubated with GM-CSF for 30 minutes before assay (priming [+]) or left unprimed until assay (priming [−]). Cells were challenged by PBS, fMLP, or C5a for 10 minutes to evoke polarization. Bars show the polarization activities of cells calculated as in Materials and Methods, after being challenged by PBS (□), fMLP (), or C5a (▪). (C and D) Polarized cells on C5a stimulation. More than 50% of the GM-treated cells (priming [+]) underwent characteristic morphological changes (C; original magnification × 150), while the IL-3–treated cells contained much fewer polarized cells with milder alterations in shape, even with priming (D; original magnification × 150).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3434/3/m_bl_0050f5d.jpeg?Expires=1767913011&Signature=pzT6u9XJHSVqs6znRfLY5DdYmeKvjrgnNeTrobA1N-QrfdmD8sngeowsvuArMhr6HudGTepQzl1VCnmO5WUMejq7cC4p1lFaxu-lGyHt459vbZwCuylqHbZSgA3cNQD24rsLyEfDKMfmNtX2t6FRBQzJa4qEbRSQ-ydA4tSlMoZ2ocDWsOaSzskW5jmcJeC~w2B7eUZnxSa6qmXHqf1hlutg9yphKCUVMiw-ajE0LdgmBYk3Gh-OOd5lwjZV260R9YBJbuB3t~a0sj6nLvaaUoHV6bn~fRTE~68md5yD6o9W19HluPx4OjrpBKwsaEk6af~yy2I-BbJ~fgUF1yfToA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
