Abstract
Gaucher disease type 1 results from the accumulation of glucocerebroside in macrophages of the reticuloendothelial system, as a consequence of a deficiency in glucocerebrosidase (GC) activity. Recent improvements in the methodologies for introducing foreign genes into bone marrow stem cells have prompted several groups to test the efficacy of gene transfer therapy as a curative treatment for Gaucher disease. Limitations of this approach include the potential for insufficient engraftment of gene-corrected cells and incomplete transduction of hematopoietic stem cells using retroviral gene transfer. Overcoming these obstacles may be critical in the case of treatment for Gaucher disease type 1, because GC transduced cells have not been shown to have a growth advantage over noncorrected cells. Here, we describe the development and application of a novel, fluorescence-activated cell sorter based assay that directly quantitates GC activity at the single cell level. In a test of this application, fibroblasts from a Gaucher patient were transduced, and high expressing cells sorted based on GC activity. Reanalysis of cultured sorted fibroblasts reveals that these cells maintain high levels of enzymatic activity, compared with the heterogeneous population from which they were sorted. The assay is sufficiently sensitive to distinguish GC activity found in Gaucher patient monocytes from that in normal controls. Furthermore, preliminary results indicate that increased GC activity can be detected in transduced, CD34+ enriched peripheral blood mononuclear cells isolated from a Gaucher patient. This method should be a useful addition to current gene therapy protocols as a means to quantitatively assess gene correction of relevant cell populations and potentially purify transduced cells for transplantation.
GAUCHER DISEASE results from mutations in the gene encoding the lysosomal enzyme glucocerebrosidase (GC) (D-glucosyl-acylsphingosine glucohydrolase, EC 3.2.1.45)1,2 and can be divided into three clinical types on the basis of the presence and severity of neurological involvement. In the case of type 1 Gaucher disease, deficient GC activity leads to an accumulation of the catabolic intermediate glucocerebroside, primarily in macrophages of the reticuloendothelial system. The common clinical manifestations of type 1 Gaucher disease, including hepatomegaly, splenomegaly, and bony lesions, result from the accumulation of enlarged macrophages or Gaucher cells in these organs. Since this disease does not include the neuronopathic symptoms present in Gaucher type 2 and type 3, bone marrow transplantation was proposed as a potential curative treatment.3 Clinical trials established allogeneic bone marrow transplantation as an effective therapy for Gaucher disease type 1.4 However, the risks inherent in this procedure, as well as the difficulties in finding compatible donors, preclude the general use of this treatment. An alternative therapeutic strategy, enzyme replacement, has recently proven to be an effective treatment.5 Critical in the development of this therapy was the finding that uptake of GC is dramatically enhanced by exoglycosidase treatment, which exposes mannose residues on the purified enzyme, allowing for efficient uptake via mannose-specific receptors on nonparenchymal liver cells.6 However, approximately 10% to 15% of treated patients develop an antibody (Ab) response to the modified enzyme.7 8 Furthermore, the requirement for repeated infusions make this therapy expensive.
Recent advances in retroviral gene transfer methodologies have prompted several groups to explore the efficacy of gene therapy as a potentially curative treatment for Gaucher disease type 1. The general protocol involves the isolation of hematopoietic stem cells (HSC) from the patient, ex vivo transduction of these cells with a recombinant retrovirus encoding the wild-type GC gene, and transplantation of the corrected cells into the patient.9 Engraftment of the transduced cells followed by differentiation into the myeloid lineages may generate sufficient levels of GC to show therapeutic efficacy. Initial experiments using the GC expression vector MFG-GC revealed that the transduction efficiency of CD34+ cells capable of bone marrow reconstitution was approximately 10% to 30%.9 Further refinement of the transduction protocol increased the transduction efficiency of colony forming unit-granulocyte macrophage (CFU-GM) to approximately 95%.10 11
While these results indicate that CD34+ cells can be efficiently transduced, high efficiency transduction of quiescent HSC has yet to be demonstrated. Consequently, in the absence of enrichment before infusion, reintroduction of nontransduced HSC may be an unavoidable consequence of retroviral gene delivery. This limitation is particularly important in the case of Gaucher cells, because transduced cells are not expected to have a proliferative advantage over nontransduced or resident hematopoietic cells. While the minimum number of corrected engrafted cells required for therapeutic efficacy is unknown, a method for the enrichment of cells with high GC activity before infusion would be a useful addition to current gene therapy strategies.
As a step toward this goal, we describe the development of a quantitative fluorescence-activated cell sorter (FACS)- based assay for the determination of relative GC activity at the single cell level. Transduced GC+ fibroblasts can be sorted on the basis of GC enzymatic activity from a heterogenous population, and expanded for further manipulation. We show that this simple assay is sufficiently sensitive to distinguish the enzymatic activity in (1) Gaucher patient CD14+ peripheral blood monocytes from that in normal controls; and (2) transduced Gaucher CD34+ cells from that in nontransduced controls. This procedure should be useful for the analysis of the efficiency of gene transfer, and for the enrichment by FACS sorting, of transduced cells before transplantation.
MATERIALS AND METHODS
Cells and tissue culture.Gaucher patient fibroblasts were transduced with the retroviral vector MFG-GC by culture in the presence of retroviral supernatant, as described.12 Fibroblasts were maintained in Minimum Essential Medium (MEM; GIBCO-BRL, Gaithersburg, MD) supplemented with 2 mmol/L L-glutamine and 20% (vol/vol) fetal calf serum (FCS; Gemini Bio-Products, Calabasas, CA). All cultures were maintained at 5% CO2 . Peripheral blood was obtained by venipuncture of volunteer donors followed by ficoll-Paque Plus (Pharmacia Biotech, Piscataway, NJ) density centrifugation under a protocol approved by the Stanford Institutional Review Board. The peripheral blood mononuclear cells (PBMCs) obtained were washed three times in RPMI 1640 (GIBCO) medium containing 10% (vol/vol) FCS and immediately processed for substrate loading. For CD34+ cell experiments, after mobilization for 10 days with granulocyte colony-stimulating factor (G-CSF) (administered at a dose of 5 μg/kg/d), peripheral blood from a Gaucher patient was isolated by daily leukapheresis over a 5-day period.13 Mobilized peripheral blood was enriched for CD34+ cells by immunoaffinity purification using the Clinical Ceprate™ column (CellPro, Inc, Bothell, WA), processed and transduced with MFG-GC, as described.13
CFU-GM and polymerase chain reaction (PCR) assays.Mobilized CD34+ enriched cells cryopreserved immediately after transduction were thawed and an aliquot was plated for CFU-GM assay, as described.13 Briefly, cells were plated at a concentration of 1 × 105 cells/mL in methylcellulose with interleukin-3 (IL-3) and GM-CSF. Day 14 CFU-GM were enumerated using an inverted microscope. Individual day 14 CFU-GM colonies were lysed, centrifuged, and the aqueous phase was processed for PCR.13 Amplification using AB1 (5′-ACG GCA TGG CAG CTT GGA TA-3′ ) and AB2 (5′-AGT AGC AAA TTT TGG GCA GG-3′ ) primers yields a unique 407-bp amplification product. Thermal cycling conditions, and electrophoresis of PCR products was conducted as described by Nimgaonkar et al.13
MUGlu cell lysate analyses.Cells were lysed in 100 mmol/L citrate buffer (pH 5.2) containing 0.1% (vol/vol) Triton X-100 and cell debris removed by centrifugation. The reaction was initiated by the addition of the synthetic substrate 4-methyl-umbelliferyl-glucopyranoside (MUGlu; Molecular Probes, Eugene, OR) to the supernatant to a final concentration of 2 mmol/L in a volume of 150 μL. The GC activator sodium taurocholate (TC) (Sigma Chemical Co, St Louis, MO) was added to a final concentration of 5.6 mmol/L, where indicated and the supernatant-substrate mix was incubated at 37°C. The reaction was stopped with the addition of 100 μL of stop buffer (15 mmol/L EDTA, 300 mmol/L Glycine, pH 11.2). Fluorescence of the product methylumbelliferone was measured in wells of a 96-well plate with a Fluoroskan II fluorescence plate reader (Flow Labs, McLean, VA) (excitation at 355 nm, emission at 460 nm).
Substrate loading and Conduritol B epoxide (CBE) inhibition studies.Gaucher fibroblasts and hematopoietic cells were pelleted and resuspended in 50 to 100 μL of staining medium (SM) (biotin and flavin deficient RPMI; GIBCO), supplemented with 4% vol/vol FCS at a concentration of 1 to 10 × 106/mL, and incubated at 37°C for 10 minutes. To stain the cells for GC activity, the cell suspension was mixed with an equal volume of 1 mmol/L 5′-chloromethylfluorescein-di-β-D-glucoside (CMFDGlu, Molecular Probes) or 5′pentafluorobenzoylaminofluorescein-di-β-D-glucoside (PFBFDGlu) (Molecular Probes, MPR 71099), dissolved in SM (also equilibrated to 37°C), and incubated at 37°C for 0 to 30 minutes. To terminate substrate loading, 3 mL of ice-cold SM was added to the cell suspension, and the cells were pelleted at 300g for 5 minutes. PBMC were processed identically, with the exception of the addition of EDTA to a final concentration of 1 mmol/L in all media to prevent monocyte loss as a result of adherence. All cells were processed for FACS analysis as described below.
Preliminary experiments were conducted using the fluorogenic GC substrate CMFDGlu. However, during the course of this work, PFBFDGlu was substituted for CMFDGlu, since this substrate is easier to synthesize and significantly more stable at −20°C than CMFDGlu. PFBFDGlu stock maintained in the dark at 50 mmol/L in 100% dimethyl sulfoxide (DMSO) is stable for several months at −20°C. Preliminary experiments indicate that these substrates behave similarly in loading experiments and are retained in cells as a result of conjugation to reactive intracellular thiols (data not shown). For GC inhibition studies, viable cells were resuspended in SM supplemented with CBE (Sigma) at a final concentration of 1 mmol/L and cultured at 37°C for 30 to 60 minutes before the addition of substrate. For chloroquine inhibition experiments, Chloroquine was added at a final concentration of 600 μmol/L during the last 5 minutes of incubation in the presence of substrate. After incubation, substrate and inhibitor were removed by centrifugation as described above.
Cell surface staining, FACS analysis, and sorting.For cell surface immunophenotyping, 1 to 2 × 106 cells were loaded with substrate and washed as described above. The cells were resuspended in 100 μL SM, and stained with the anti-CD14 monoclonal antibody (MoAb) leuM3 or anti-CD34 conjugated to phycoerythrin (Becton Dickinson, San Jose, CA) for 20 minutes at 4°C. Fibroblasts and hematopoietic cells were resuspended in 100 to 400 μL of ice-cold SM supplemented with propidium iodide at a final concentration of 1 μg/mL. Cells were maintained on ice throughout the FACS analyses to minimize efflux of the fluorescein product. In all FACS analyses, dead cells were excluded by electronic gating on the basis of propidium iodide staining.
Viable Gaucher fibroblasts were sorted into 100% FCS. After sorting, the medium was brought to a final concentration of 20% FCS by the addition of supplemented MEM, and the sorted cells were cultured as described above. Sorting and analysis was done on a FACSTAR Plus (Becton Dickinson). Multiparameter data were collected and analyzed as described14 by using FACS-Desk software at the Stanford Shared FACS Facility.
RESULTS
Substrate internalization kinetics.We previously showed that fluorescein-based hydrolase substrates can be efficiently introduced into the cytosol of mammalian cells.14 Subsequently, we tested several bacterial glucosidases against glucose-based substrates,15 establishing the utility of such dyes in FACS studies. Based on these observations, we chose to apply this methodology to the human GC enzyme. Mature GC is primarily localized in lysosomes.16 We reasoned that modifying the loading conditions to allow for substrate uptake by pinocytosis would target the substrate to the lysosomal compartment, maximizing exposure to the catalytically active GC enzyme.17 We incubated nontransduced Gaucher fibroblasts or fibroblasts transduced with the MFG-GC retrovirus in the presence of the fluorogenic GC substrate CMFDGlu for 2 to 30 minutes and analyzed the loaded cells by FACS using conventional fluorescein emission optics.
While FACS analysis reveals that CMFDGlu loaded transduced fibroblasts show brighter median fluorescence (MF ) levels than nontransduced fibroblasts at all incubation time points (Fig 1A), the fluorescence distributions show minimal overlap with a 10-minute incubation. Plotting of the MF values of transduced versus nontransduced cells reveals that the fluorescence ratio (transduced minus background/nontransduced minus background MF values) increases with increasing incubation time, reaching a maximum value of 14.0 with a 10-minute incubation (Fig 1B). With longer incubation times, however, the fluorescence distributions increasingly overlap, and the MF ratio decreases to 3.6 with a 30-minute incubation. These results indicate that the rate of substrate hydrolysis of the MFG-GC transduced population plateaus and then decreases, perhaps as a result of substrate exhaustion. Since the nontransduced cells hydrolyze substrate at a slower rate, substrate exhaustion becomes apparent only with longer incubation times (data not shown). Thus, for these fibroblasts, a 10-minute exposure to CMFDGlu appears to be optimal for distinguishing transduced from nontransduced cells.
FACS analysis of CMFDGlu-loaded Gaucher fibroblasts or PFBFDGlu-loaded Gaucher PBMC. MFG-GC transduced and nontransduced Gaucher fibroblasts (A) or Gaucher and control PBMC (C), were incubated at 37°C for 5 to 30 minutes in the presence of 1 mmol/L CMFDGlu (for 5, 10, or 30 minutes) or PFBFDGlu (for 5, 15, or 30 minutes), respectively. The fluorescence histograms of fibroblasts or large PBMC (FS, OS scatter high) for each time point are overlaid to indicate the relative fluorescence levels of each pair of samples (upper panel, Gaucher fibroblasts: — nontransduced, — transduced; lower panel, FS, OS large PBMC isolated from: — Gaucher patients, or — controls). The MF values of nontransduced fibroblasts or Gaucher patient FS, OS large cells are shown in the upper left corner of each histogram. The MF values of transduced fibroblasts or control FS, OS large cells are shown in the upper right corner. The MF values are plotted versus time in (B) and (D) for each of the samples displayed in A and C, respectively. The MF ratio is determined by subtracting the MF of cells incubated in the absence of substrate, and taking the ratio of the corrected MF values.
FACS analysis of CMFDGlu-loaded Gaucher fibroblasts or PFBFDGlu-loaded Gaucher PBMC. MFG-GC transduced and nontransduced Gaucher fibroblasts (A) or Gaucher and control PBMC (C), were incubated at 37°C for 5 to 30 minutes in the presence of 1 mmol/L CMFDGlu (for 5, 10, or 30 minutes) or PFBFDGlu (for 5, 15, or 30 minutes), respectively. The fluorescence histograms of fibroblasts or large PBMC (FS, OS scatter high) for each time point are overlaid to indicate the relative fluorescence levels of each pair of samples (upper panel, Gaucher fibroblasts: — nontransduced, — transduced; lower panel, FS, OS large PBMC isolated from: — Gaucher patients, or — controls). The MF values of nontransduced fibroblasts or Gaucher patient FS, OS large cells are shown in the upper left corner of each histogram. The MF values of transduced fibroblasts or control FS, OS large cells are shown in the upper right corner. The MF values are plotted versus time in (B) and (D) for each of the samples displayed in A and C, respectively. The MF ratio is determined by subtracting the MF of cells incubated in the absence of substrate, and taking the ratio of the corrected MF values.
Specificity of hydrolysis.To determine what fraction of substrate hydrolytic “activity” in nontransduced Gaucher fibroblasts is due to residual GC activity, we tested the susceptibility of PFBFDGlu hydrolysis to the specific GC inhibitor CBE18,19 (PFBFDGlu was used in place of CMFDGlu in this and subsequent experiments; see Materials and Methods). CBE effectively inhibits GC activity in cultured fibroblasts,20 but has no effect on cell viability or endocytosis.21 Addition of 1 mmo/L CBE, before the addition of PFBFDGlu, inhibited GC activity in nontransduced or transduced fibroblast cultures 11-fold or sevenfold, respectively (Fig 2A). The hydrolytic activity of nontransduced Gaucher fibroblasts was inhibited to near background levels by CBE, suggesting that residual GC activity is primarily responsible for substrate hydrolysis. These results are consistent with lysate analyses of Gaucher cells using the MUGlu substrate.22 23
Properties of substrate hydrolysis and product retention in fibroblasts expressing heterogeneous levels of GC. Fluorescence data on 10,000 viable cells was collected by FACS, as described, and is displayed in histogram form. The fluorescence profiles of fibroblasts incubated in the absence of substrate are shown for each experiment. (A) To determine the role of GC in PFBFDGlu hydrolysis, nontransduced, or MFG-GC transduced fibroblasts were incubated in the presence of 1 mmol/L CBE for 30 minutes at 37°C before incubation in the presence of 1 mmol/L PFBFDGlu. The MF values of each population are included above each histogram. (B) To determine the pH sensitivity of substrate hydrolysis, nontransduced or sorted MFG-GC transduced cells were incubated in the presence or absence of 600 μmol/L chloroquine during the last 5 minutes of 1 mmol/L CMFDGlu loading, as described above. (C) Fluorescence histograms of nontransduced, sorted MFG-GC transduced, or a 7.5:1 mix of nontransduced and sorted MFG-GC transduced cells incubated in the presence of 1 mmol/L CMFDGlu. Note: data shown in B and C was collected in the same experiment.
Properties of substrate hydrolysis and product retention in fibroblasts expressing heterogeneous levels of GC. Fluorescence data on 10,000 viable cells was collected by FACS, as described, and is displayed in histogram form. The fluorescence profiles of fibroblasts incubated in the absence of substrate are shown for each experiment. (A) To determine the role of GC in PFBFDGlu hydrolysis, nontransduced, or MFG-GC transduced fibroblasts were incubated in the presence of 1 mmol/L CBE for 30 minutes at 37°C before incubation in the presence of 1 mmol/L PFBFDGlu. The MF values of each population are included above each histogram. (B) To determine the pH sensitivity of substrate hydrolysis, nontransduced or sorted MFG-GC transduced cells were incubated in the presence or absence of 600 μmol/L chloroquine during the last 5 minutes of 1 mmol/L CMFDGlu loading, as described above. (C) Fluorescence histograms of nontransduced, sorted MFG-GC transduced, or a 7.5:1 mix of nontransduced and sorted MFG-GC transduced cells incubated in the presence of 1 mmol/L CMFDGlu. Note: data shown in B and C was collected in the same experiment.
Observation by fluorescence microscopy revealed an appreciable level of CBE inhibitable punctate fluorescein fluorescence in PFBFDGlu loaded fibroblasts, indicating that endosomal/lysosomal GC is largely responsible for substrate hydrolysis (data not shown). The presence of lysosomal enzymes, including GC, in the endosomal compartment has been reported previously.24,25 To further establish the role of endosomal/lysosomal GC in substrate hydrolysis, we tested the effect of chloroquine on GC activity in viable cells. GC shows optimal activity at a pH of approximately 5.5, with reduced activity at relatively basic pH.22 Culture of intact lymphoblasts or macrophages in the presence of the lysosomatropic weak bases, such as NH4Cl or chloroquine,17 leads to an increase in endosomal pH and a concomitant decrease in the activity of GC.22 FACS analysis of nontransduced or transduced viable Gaucher fibroblasts revealed that preincubation in the presence of 300 μmol/L chloroquine inhibited substrate hydrolysis 10-fold or sevenfold, respectively, compared with chloroquine free cultures (Fig 2B). As the absorbance and emission peak heights of fluorescein are considerably enhanced at basic pH (pKa = 6.1 in the context of the substrates used here), the apparent incomplete inhibition by chloroquine may be an artifact resulting from increased fluorescence from the relatively low levels of fluorescein in the neutralized lysosomal compartment. Thus, the enzymatic activity detected by the FACS assay shows pH sensitivity comparable with that previously described for GC.22 Taken together, these results indicate that the fluorescein-based substrates are pinocytosed and hydrolyzed by endosomal/lysosomal GC.
Sorting of transduced fibroblasts.To test whether cells could be selected on the basis of GC activity, nontransduced or transduced fibroblasts were stained as described above with CMFDGlu and analyzed by FACS. Sorting gates were chosen such that cells in the transduced population with fluorescein fluorescence levels overlapping those of nontransduced cells were excluded (Fig 3A). Reanalysis of the sorted population by FACS 34 days later revealed that the sorted cells maintained approximately the same relative increase in activity compared with nontransduced cells as they did on the day of sorting (10-fold v eightfold, respectively). To independently measure the levels of GC activity in the sorted cells, as well as in the transduced population from which they were sorted, a lysate assay using the MUGlu substrate was used (Fig 3B). While transduced cells show twofold greater activity than nontransduced cells, sorted cells showed greater than fivefold more activity than nontransduced cells. Furthermore, immunohistochemical analyses 26 revealed an increase in GC protein in the sorted population (data not shown), conclusively demonstrating that cells expressing relatively high levels of GC were enriched by the FACS assay and sorting.
Analysis of sorted, transduced Gaucher fibroblasts by flow cytometry and by the MUGlu lysate assay. (A) After loading in the presence of 1 mmol/L CMFDGlu, 30,000 cells were sorted on the basis of fluorescein fluorescence and cultured for further analysis. The threshold chosen for sorting excluded all transduced cells with fluorescence values overlapping those of the nontransduced population. The sorted population was reanalyzed by FACS 34 days later. Relative fluorescence levels are given for each sample and were calculated by setting the median fluorescence value of the nontransduced population in each experiment to 1.0. (B) Forty days postsorting, 30,000 cells were harvested, lysed, and the relative GC activity was measured using the MUGlu assay. Mean Methylumbelliferone fluorescence levels are shown for the nontransduced, transduced, and sorted populations. Lysate analysis was conducted in triplicate.
Analysis of sorted, transduced Gaucher fibroblasts by flow cytometry and by the MUGlu lysate assay. (A) After loading in the presence of 1 mmol/L CMFDGlu, 30,000 cells were sorted on the basis of fluorescein fluorescence and cultured for further analysis. The threshold chosen for sorting excluded all transduced cells with fluorescence values overlapping those of the nontransduced population. The sorted population was reanalyzed by FACS 34 days later. Relative fluorescence levels are given for each sample and were calculated by setting the median fluorescence value of the nontransduced population in each experiment to 1.0. (B) Forty days postsorting, 30,000 cells were harvested, lysed, and the relative GC activity was measured using the MUGlu assay. Mean Methylumbelliferone fluorescence levels are shown for the nontransduced, transduced, and sorted populations. Lysate analysis was conducted in triplicate.
To establish whether this assay is sufficiently sensitive to discriminate between different levels of GC activity within a transduced population, transduced fibroblasts incubated in the presence of CMFDGlu were sorted with gates chosen to obtain relatively dull, intermediate, and bright fluorescent fractions (data not shown). Each sorted population was immediately lysed and analyzed with the MUGlu assay, yielding low, intermediate, and high activity fractions, respectively. Taken together, these results indicate that fluorescein fluorescence, as measured by FACS, can be used as an accurate indicator of relative GC activity.
Sorting of a population of cells with high levels of GC allowed us to explicitly establish that cells with high GC activity could be distinguished from those with lower activity even when the former are present at a low frequency, a requirement for sorting from populations transduced at low efficiency. The sorted MFG-GC transduced cells described in Fig 3 were mixed with nontransduced Gaucher fibroblasts at a ratio of 1:7.5 and analyzed by FACS (Fig 2C). Comparison of the fluorescence histograms of each population revealed that the mixed population includes a “shoulder” of relatively highly fluorescent cells showing similar fluorescence to the sorted population alone. Furthermore, software analysis of the data reveals that the bright cells comprise 12.5% of the mixed population, close to the predicted fraction of 11.8%. These results indicate that there is minimal transfer of the fluorescein product between cells, and that this assay can be used to distinguish the relatively high GC activity generated in transduced fibroblasts from the residual GC activity present in nontransduced Gaucher fibroblasts.
Analysis of Gaucher patient versus control PBMC.Our initial results with transformed fibroblasts prompted us to test whether the method could be adopted for use with peripheral blood cells. PBMC isolated from a patient previously diagnosed with Type 1 Gaucher disease and a normal control were isolated by ficoll-hypaque and a loading experiment was conducted as described for the fibroblasts to establish the kinetics of substrate hydrolysis (Fig 1C). A minor population of cells (15% to 30% of PBMC) with relatively high forward (FS) and orthogonal light scatter (OS) profiles showed a significant time dependent increase in fluorescein fluorescence. With a 30-minute incubation, the difference in control versus Gaucher MF values reached a maximum of 4-fold for this population (Fig 1D). With a 45-minute incubation, a fraction of the control cells show fluorescence levels beyond the quantifiable range of the FACS (data not shown). Thus, a 30-minute incubation time was chosen for all further PBMC experiments.
The relatively high light scatter phenotype of the fluorescein bright population is characteristic of the monocyte fraction of PBMC. To determine the type(s) of leukocyte present in the fluorescein-bright population, substrate-loaded PBMC were characterized by cell surface immunofluorescence staining. A MoAb recognizing CD14, a marker present on the majority of myeloid-lineage cells in peripheral blood,27,28 stained greater than 95% of the large, fluorescein bright mononuclear cells (data not shown). These results indicate that CD14+ cells have higher GC activity, and/or higher endocytic capacity than lymphocytes, both properties that have previously been reported for monocytes.29 30 Addition of chloroquine 20 minutes before the addition of PFBFDGlu inhibited the hydrolytic activity detected in the CD14+ population approximately 10-fold (data not shown), indicating that endosomal/lysosomal GC is responsible for the intracellular hydrolysis of PFBFDGlu in PBMC.
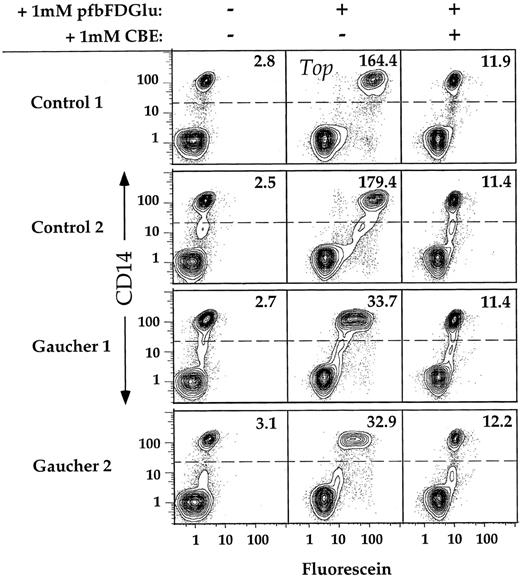
We next tested whether the FACS assay is sufficiently sensitive to consistently distinguish GC activity in Gaucher type 1 patient monocytes from that detected in control monocytes. The results of a typical experiment are shown in Fig 4. In the absence of substrate, the median fluorescein fluorescence value calculated for monocytes (CD14+) isolated from two unrelated Gaucher patients was not significantly different from that of normal controls. However, when incubated for 30 minutes in the presence of PFBFDGlu, the monocyte cells isolated from both patients were significantly less fluorescent than those isolated from normal individuals. In the presence of PFBFDGlu and the inhibitor CBE, both patient and control samples show similar fluorescence distributions (Fig 4), suggesting that the remaining fluorescence is due to hydrolytic activity independent of GC, or to a fluorescent contaminant in the substrate preparation, or both.
Discrimination between CD14+ cells isolated from controls or Gaucher patients. PBMC were isolated from two controls and two unrelated Gaucher patients, incubated for 30 minutes in the presence of 1 mmol/L PFBFDGlu, and stained with αCD14 MoAb. Preincubation in the presence of the specific inhibitor CBE was conducted for 1 hour, as described. Dead cells were excluded by software gating on the basis of PI staining. Greater than 90% of cells were viable in all samples analyzed. Ten percent probability contour plots of CD14 versus fluorescein are shown for PBMC incubated in the presence or absence of substrate. The MF values of the CD14+ populations within each sample are displayed in the upper right corner of each plot.
Discrimination between CD14+ cells isolated from controls or Gaucher patients. PBMC were isolated from two controls and two unrelated Gaucher patients, incubated for 30 minutes in the presence of 1 mmol/L PFBFDGlu, and stained with αCD14 MoAb. Preincubation in the presence of the specific inhibitor CBE was conducted for 1 hour, as described. Dead cells were excluded by software gating on the basis of PI staining. Greater than 90% of cells were viable in all samples analyzed. Ten percent probability contour plots of CD14 versus fluorescein are shown for PBMC incubated in the presence or absence of substrate. The MF values of the CD14+ populations within each sample are displayed in the upper right corner of each plot.
PBMC were obtained from a total of six unrelated Gaucher type 1 patients and seven controls, and analyzed by flow cytometry in four independent experiments (Fig 5). Generally, freshly isolated PBMC from two patient samples and a minimum of two control samples were analyzed in each experiment. By subtracting the MF of the monocytes incubated in the presence of CBE from the MF of the monocytes incubated in the absence of CBE for each individual, the “GC specific” contribution of PFBFDGlu hydrolysis was determined. To compare analyses conducted on separate days, the CBE correction was conducted, and the mean of the resulting “GC specific” value of the control samples was determined. The activities of all samples were normalized by dividing by this value (eg, [164.4 − 11.9] = 152.5; [179.4 − 11.4] = 168.0; 168.0 + 152.5/2 = 160.3; 152.5/160.3 = .95, 168.0/160.3 = 1.05, etc). The small standard deviation (±10%) of normalized control MF values reveals that enzymatic activity as determined by FACS is homogeneous among normal individuals. The mean corrected fluorescein fluorescence of Gaucher patient samples was 19% (SD ± 7%) of that determined for control samples (P < .002), indicating that the FACS assay is sufficiently sensitive and reproducible to distinguish GC activities in Gaucher and normal monocytes.
Summary of normalized median fluorescence (MF ) values of monocytes (CD14+) isolated from Gaucher patients or normal controls. PBMC obtained in four separate experiments from six unrelated patients and seven controls were collected, processed, and analyzed by FACS as described in Fig 4. CBE inhibition was carried out in parallel to determine the GC specific contribution for each sample. The normalized median fluorescein fluorescence values of monocytes are shown for all samples. Three of the control samples (•) were collected from a single individual on 3 different days. To compare analyses conducted on separate days. The CBE correction was conducted, and the mean of the resulting “GC specific” value of the control samples was determined. The activities of all samples were normalized by dividing by this value. The standard deviation of normalized control MF values was ±10%. The mean corrected fluorescein fluorescence of Gaucher patient samples was 19% (SD ± 7% of that determined for control samples (P < .002). Statistical analysis was conducted with JMP software using the Mann-Whitney U test.
Summary of normalized median fluorescence (MF ) values of monocytes (CD14+) isolated from Gaucher patients or normal controls. PBMC obtained in four separate experiments from six unrelated patients and seven controls were collected, processed, and analyzed by FACS as described in Fig 4. CBE inhibition was carried out in parallel to determine the GC specific contribution for each sample. The normalized median fluorescein fluorescence values of monocytes are shown for all samples. Three of the control samples (•) were collected from a single individual on 3 different days. To compare analyses conducted on separate days. The CBE correction was conducted, and the mean of the resulting “GC specific” value of the control samples was determined. The activities of all samples were normalized by dividing by this value. The standard deviation of normalized control MF values was ±10%. The mean corrected fluorescein fluorescence of Gaucher patient samples was 19% (SD ± 7% of that determined for control samples (P < .002). Statistical analysis was conducted with JMP software using the Mann-Whitney U test.
This level of residual activity was higher than that obtained with the MUGlu lysate assay conducted in parallel in the presence of the GC “activator” TC, in which both Gaucher patients showed <10% of the activity of normal control PBMC (data not shown). This inconsistency may be explained by the fact that the level of residual GC activity is highly dependent on the conditions used in the lysate assay.22,31 32 As the FACS assay is conducted with living cells, the residual activity detected may be more physiologically relevant than that determined in the presence of the various activators and inhibitors used in the lysate assay.
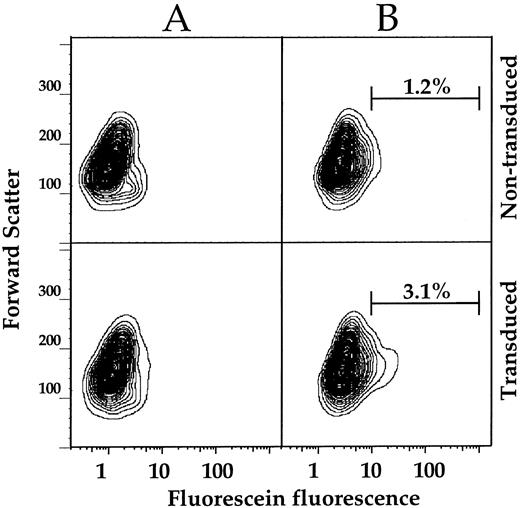
Analysis of mobilized CD34+ Gaucher cells.To determine whether the FACS assay is sufficiently sensitive to detect GC activity in gene-corrected CD34+ enriched Gaucher PBMC, nontransduced and MFG-GC transduced Gaucher patient CD34+ cells were analyzed by FACS or plated for CFU-GM assay.13 Both cell types show low levels of autofluorescence when incubated at 37°C in the absence of substrate (Fig 6A). FACS analysis of cells incubated in the presence of CMFDGlu revealed that 3.1% of transduced cells showed GC activity greater than 98.8% of nontransduced cells (Fig 6B). Thirty-six percent of the transduced cells remained CD34+ with 2.0% of these cells having GC activity greater than 99.4% of the CD34+ cells in the nontransduced sample (data not shown). We also observed a significant proportion of cells in the CD34− fraction of the transduced sample with higher GC activity than the comparable fraction of the untransduced sample (3.6% showed higher GC activity than 98.5% of the untransduced CD34− cells) (data not shown). These GC+ CD34− cells are most likely a mixture of CD34+ hematopoietic progenitors that have differentiated and lost CD34 expression and CD34− cells that contaminated the initial CD34 isolate.
FACS analysis of mobilized CD34+ enriched PBMC transduced with MFG-GC. Nontransduced (top panels) or MFG-GC transduced (bottom panels) CD34+ enriched PBMC were incubated at 37°C for 10 minutes in the absence (A) or presence (B) of 1 mmol/L CMFDGlu. Following substrate loading, FACS analysis was conducted as described for Gaucher fibroblasts. To approximate the fraction of GC positive cells in the transduced sample, logarithmic gating was applied to the substrate loaded samples. Fifty-thousand events were collected per sample. Results are displayed as 2% probability contour plots. Substrate loading did not decrease viability as measured by PI staining (data not shown).
FACS analysis of mobilized CD34+ enriched PBMC transduced with MFG-GC. Nontransduced (top panels) or MFG-GC transduced (bottom panels) CD34+ enriched PBMC were incubated at 37°C for 10 minutes in the absence (A) or presence (B) of 1 mmol/L CMFDGlu. Following substrate loading, FACS analysis was conducted as described for Gaucher fibroblasts. To approximate the fraction of GC positive cells in the transduced sample, logarithmic gating was applied to the substrate loaded samples. Fifty-thousand events were collected per sample. Results are displayed as 2% probability contour plots. Substrate loading did not decrease viability as measured by PI staining (data not shown).
Analysis of individual day 14 CFU-GM colonies by PCR revealed a transduction frequency of 87% (data not shown). There are several potential explanations for the disparity between the number of transduced cells as measured by FACS versus by PCR of CFU-GM, including: (1) colonies detected in the CFU-GM assay are enriched for a subset of cells that are transduced at higher frequency than the population from which they are derived; (2) retroviral mediated GC expression in the majority of transduced cells is insufficient to allow for discrimination from nontransduced cells under the experimental conditions applied; or (3) fluid phase pinocytosis in undifferentiated hematopoietic cells is insufficient to allow for adequate internalization of the substrate. Nevertheless, the direct observation of higher GC activity cells in the CD34+ fraction of transduced cells suggests that the FACS assay for GC activity can be applied to the detection of transduced hematopoietic progenitor cells.
DISCUSSION
Initial efforts to establish the efficacy of retroviral mediated gene transfer and the stability of GC expression focused on the delivery of the human GC gene into murine stem cells.33-36 The efficiency of transduction was determined by PCR of CFU-GM, and the level of GC expression by immunohistochemical, or cell lysate analyses. Long-term stable expression of the human enzyme in the murine system prompted us to develop a methodology for human CD34 cell transduction, in anticipation of using these cells as targets for gene therapy.9-11,37 While the PCR assay can be used in human studies, the potential presence of normal levels of GC protein in patients with Gaucher disease22 32 precludes the use of the immunohistochemical assay. As a result, the only procedure available for clinical analysis of GC activity is the MUGlu bulk cell lysate assay, which cannot be used to determine the distribution of GC activity, or the presence and number of rare cells with GC activity, in heterogeneous populations such as PBMC. The quantitative assay described here, allowing for the detection of GC activity on a single cell basis, will be useful in the prognosis of the efficacy of gene therapy, and potentially in the development of improved gene therapy protocols.
The use of fluorogenic enzymatic substrates in living cells was first described by Rotman and Papermaster.38 Subsequently, the utility of this esterase assay was established by a number of groups.39,40 Previously, we developed two FACS-based reporter enzyme assays using the Eschericula coli hydrolase genes β-galactosidase14 and β-glucuronidase.15 These flow cytometry based assays allow quantitation of relative enzymatic activity at the single cell level on statistically significant numbers of cells, as well as for the isolation of cells by sorting on the basis of this activity. The successful application of the β-gal assay in several experimental systems,41-44 as well as the demonstration of lysosomal uptake of fluorescein-based compounds in murine macrophages,17 prompted us to test the potential utility of this methodology in the study of human diseases resulting from deficiencies of specific hydrolases. Recently, Yeyati et al45 established a FACS-based selection system for the enzymatic deficiency responsible for Niemann-Pick disease. Since GC is known to cleave a number of synthetic β-glucosides,46 we chose initially to apply our method to the study of this lysosomal hydrolase.
While there are other means of enriching for transduced cells ex vivo, including drug selection systems, selection on the basis of drug resistance has not been adopted for use in the clinic for several reasons. Retroviral vectors containing additional promoters or enhancers necessary to drive expression of a drug-selectable gene generally show lower viral titers and lower levels of mRNA than constructs with just the viral LTR.47 While cells transduced with a vector encoding a drug selection marker are theoretically resistant, some cell types may be more susceptible to the toxic effects of the administered drug. Furthermore, since the ex vivo conditions for maintenance may not accurately reflect the in vivo milieu, the temporal requirement for effective drug selection in culture increases the potential for differentiation of harvested bone marrow cells, and thus loss of self-renewing pluripotent HSC. The recent development of bicistronic vectors encoding cell surface markers as a means of selection via cell sorting circumvents the problems associated with drug selection.48 However, the introduction of xenogeneic proteins may adversely influence the biological function of HSC, and may render the cells immunogenic.49
The assay described here is nontoxic, and permits the rapid enrichment of gene corrected cells based on GC enzymatic activity. Selection of transduced cells by FACS requires that the transduced cells have pinocytic activity, and express levels of GC sufficient for hydrolysis of substrate at levels necessary for detection. Preliminary experiments with mobilized CD34+ enriched PBMC indicate that a fraction of transduced cells can be distinguished from nontransduced cells by FACS. However, the relative fluorescence levels detected in these cells is clearly diminished compared with transduced fibroblasts. Medin et al reported similar results for surface expression of a surrogate marker encoded by a Moloney based retroviral vector.48 As surface phenotyping does not require pinocytosis, the reduced signal to noise ratio is probably due to a relatively low level of retroviral vector mediated GC expression in CD34+ cells. If enzymatic activity is the limiting factor, extending the incubation time after substrate loading to allow further substrate hydrolysis may increase the sensitivity of the assay. Given a fluorescein positive fraction of 3.1%, a sorting efficiency of 75%, and a flow rate of 4,000 cells/second, approximately 106 fluorescent cells could be sorted in 3 hours. As 1.2% of nontransduced cells were scored as fluorescein positive, an enrichment of GC positive cells to 60% of the total sorted would be predicted using the conditions described in Fig 6. Clearly, establishing conditions that enhance the signal to noise ratio would improve the enrichment of the sorted population and the rate at which corrected cells could be sorted. Recent developments in high-speed cell sorting of hematopoietic stem/progenitor cells50 suggests that enrichment of gene corrected cells for transplantation purposes may be feasible.
Recently we tested whether this assay can be used to measure the activity of β-glucuronidase, a hydrolase responsible for the lysosomal storage disorder, Mucopolysaccharidosis type VII. Using a murine model system of this disorder51 and a substrate similar to that used in the FACS-GC assay, we developed a similar method to measure lysosomal β-glucuronidase (Gus) activity by FACS. As for the FACS-GC assay, fibroblasts transduced with a Gus expression vector52 can be distinguished from nontransduced cells based on Gus activity, and sorted for further analysis (manuscript in preparation). Thus, the FACS assay described here may be applicable to hydrolase deficiencies in general, and may be a useful addition to gene therapy protocols for such genetic diseases.
ACKNOWLEDGMENT
The authors are grateful to Garry Nolan for his constant support and helpful suggestions on applications of the novel fluorogenic substrates used in this project. We also thank Mike Anderson, Rachel Gerstein, Mario Roederer, Peter Katsikis, and Garry Nolan for advice during the course of this work, and for critical review of the manuscript. We thank Chris Fanger and Trina Mohney for technical help. We are especially grateful to Richard Haugland at Molecular Probes, Inc for the creation and provision of the substrates used in this report. We also thank Jixiang Liu (Molecular Probes) for the preparation of fluorogenic substrates used in some experiments described in this manuscript.
Supported in part by National Institutes of Health (NIH) Grant No. CA42509 to L.A.H., NIH training Grant No. GM07790 to M.L., NIH Grants No. DK48436, DK44935, and RR00056-35 and a grant from Genzyme Corporation to J.A.B.
Address reprint requests to William G. Kerr, PhD, Department of Molecular and Cellular Engineering, Institute for Human Gene Therapy, University of Pennsylvania, Room 405, Stellar-Chance Laboratories, 422 Curie Blvd, Philadelphia, PA 19104-6100.