Abstract
The influence of busulfan (BU) plasma concentration on outcome of transplantation from HLA identical family members for the treatment of chronic myelogenous leukemia (CML) was examined in 45 patients transplanted in chronic phase (CP) (n = 39) or accelerated phase (AP) (n = 6). All patients received the same regimen of BU, 16 mg/kg orally and cyclophosphamide (CY), 120 mg/kg intravenously. Plasma concentrations of BU at steady state (CSSBU) during the dosing interval were measured for each patient. The mean CSSBU was 917 ng/mL (range, 642 to 1,749; median, 917; standard deviation, 213). Of patients with CSSBU below the median, seven (five of 18 in CP and two of four in AP) developed persistent cytogenetic relapse and three of these patients died. There were no relapses in patients with CSSBU above the median. The difference in the cumulative incidence of relapse between the two groups was statistically significant (P = .0003). CSSBU was the only statistically significant determinant of relapse in univariable or multivariable analysis. The 3-year survival estimates were 0.82 and 0.64 for patients with CSSBU above and below the median (P = .33). There was no statistically significant association of CSSBU with survival or nonrelapse mortality, although the power to detect a difference in survival between 0.82 and 0.64 was only 0.24, similarly CSSBU above the median was not associated with an increased risk of severe regimen-related toxicity. We conclude that low BU plasma levels are associated with an increased risk of relapse.
ALLOGENEIC MARROW transplantation from HLA identical siblings is the most effective treatment for chronic myelogenous leukemia (CML).1-4 The results of transplantation have improved over the past 10 years,4-7 and the best results are obtained when patients are transplanted early during chronic phase (CP). In a recent Seattle study,8 the 4-year probability of survival for 128 patients transplanted within 1 year of diagnosis after conditioning with either busulfan and cyclophosphamide (BU + CY) or cyclophosphamide and total body irradiation (CY + TBI) was 0.86.
In a prospective randomized study9 of marrow transplantation for patients with CML in CP, we demonstrated that the BU + CY regimen was better tolerated than and associated with survival and relapse probabilities, which compared favorably to the CY + TBI regimen. All patients received methotrexate (MTX) and cyclosporine (CSP) as prophylactic immunosuppression against acute graft-versus-host disease (GVHD). Since November 1992, all patients older than 14 years with CML in CP or accelerated phase (AP) receiving transplants from HLA-identical related donors have been conditioned with the BU + CY + MTX + CSP regimen and through 1995, we have transplanted 168 patients in CP using this regimen.
It has been demonstrated that patients receiving oral BU achieve widely varying plasma concentrations and that low concentrations predispose to graft rejection,10 while high levels increase the risk of toxicity.10,11 The antileukemic effect of the BU + CY regimen putatively resides largely in BU, and it is possible that higher levels will protect against relapse of CML. It is important to evaluate this possibility because variations in BU plasma concentration can be overcome with a high degree of accuracy by individually adjusting the oral dose to achieve a desired target concentration.12
In the present report, we evaluate the influence of plasma BU levels on relapse and survival in patients transplanted for the treatment of CML at the Fred Hutchinson Cancer Research Center (Seattle, WA) using a BU + CY regimen.
MATERIALS AND METHODS
Patient Eligibility and Accrual
Between 1991 and 1995, BU pharmacokinetics were studied in 45 patients with CML in CP (n = 39) or AP (n = 6) undergoing marrow transplantation from HLA-identical family member donors. Patients were selected on the basis of the availability of laboratory resources and all patients selected are included in this report. The diagnosis of CML was established by standard criteria: all patients were Ph chromosome positive and were categorized as being in CP or AP on the basis of previously defined characteristics.4 Disease and phase status were confirmed immediately before transplantation.
Informed Consent
Risks of the treatment protocols were fully explained to the patients, donors, and relatives. Informed consent was obtained using forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Treatment Regimen
All patients in this study were conditioned for marrow transplantation with a regimen consisting of BU 1.0 mg/kg administered orally four times daily on each of 4 successive days (total dose, 16 mg/kg) followed by 60 mg/kg of CY administered intravenously on each of 2 successive days (total dose, 120 mg/kg). BU was administered as 2-mg tablets in gelatin capsules. Antiemetics were given in accordance with our standard practice, which includes ondansetron 8 mg orally before each BU dose, diphenhydramine 25 to 50 mg every 4 to 6 hours as needed, and lorazepam 0.5 to 2.0 mg every 4 to 6 hours as needed. Phenytoin was administered to all patients in accordance with our standard protocol, which includes a loading dose of 15 mg/kg divided among three doses before BU administration and maintenance dosing of 300 mg orally daily continued until 24 hours after the last dose of BU.
Prophylaxis against acute GVHD consisted of MTX and CSP.13,14 All patients were scheduled to receive intravenous MTX 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. CSP was administered intravenously at a dose of 3 mg/kg per day in two divided doses starting on the day before marrow infusion (day −1). Oral CSP, 12.5 mg/kg per day was substituted for intravenous administration when tolerated. Starting on day 50, oral CSP administration was tapered by 5% weekly and discontinued on day 180. Doses of CSP were adjusted if necessary (usually because of renal or hepatic dysfunction), and MTX administration was adjusted for severe mucositis, extravascular fluid accumulation, or impairment of renal function. Acute and chronic GVHD were diagnosed and graded using established criteria.15 Acute GVHD was treated with prednisone, antithymocyte globulin, or monoclonal antibodies.16,17 Chronic GVHD was treated with prednisone alone or with CSP.18 19
Tissue Typing Studies
Forty-three donors were HLA genotypically identical siblings, one was a phenotypically identical father, and one was a phenotypically identical son, of the recipients as shown by serologic typing for HLA-A, B, DR, and DQ testing in mixed lymphocyte culture assays (recently replaced by typing for DRB and DQB alleles by hybridization of sequence specific oligonucleotide probes to PCR amplified DNA).20-22
Pharmacokinetic Studies
Details of the chemical (gas chromatography/mass spectrometry) and pharmacokinetic analyses have been described.10 To provide accurate assessments of BU exposure during conditioning, blood samples were collected at 0, 1, 2, 4, and 6 hours after any two of the morning doses on days 2, 3, or 4 of BU administration (ie, the fifth, ninth, and 13th doses). CSSBU is the ratio of BU area under the curve (AUC) over the dosing interval to the time between doses (6 hours). The CSSBU evaluated as a determinant of outcome was the mean of the values observed in each patient.
Regimen-Related Toxicity
Regimen-related toxicity was scored using the criteria described by Bearman et al.23 The overall grade assigned was the maximum grade for the bladder, renal, pulmonary, hepatic, central nervous system (CNS), mucosal, and gastrointestinal organ systems during the first 28 days posttransplant.
Engraftment and Rejection
Evidence of graft rejection was sought in patients who relapsed. When a gender difference existed between donor and patient, in situ Y chromosome hybridization24 was performed on bone marrow and peripheral blood mononuclear cells stimulated with phytohemagglutinin. When patient and donor were of the same gender, DNA from bone marrow and peripheral blood mononuclear cells was amplified for several variable number tandem repeat (VNTR) loci. The amplified fragments were examined to identify informative host or donor markers.25
Relapse
All patients were scheduled to have marrow samples examined by cytogenetic analysis on days 28, 56, 84, at 6-month intervals for 2 years, and then annually. One patient, who died with venoocclusive disease of the liver on day 34, did not have any posttransplant cytogenetic evaluations and could not be evaluated for relapse. Relapse was defined as the detection of Ph positive metaphases in the marrow after day 50 posttransplant. Relapse was further categorized as transient when Ph positive metaphases cleared from the marrow and subsequently remained undetectable without definitive therapeutic intervention. Otherwise, relapses were defined as persistent.
Infection
Blood samples were cultured weekly for the presence of cytomegalovirus (CMV). Interstitial pneumonia (IP) was diagnosed by culture, histology or histochemistry of bronchoalveolar lavage, open lung biopsy, or autopsy.
Various infectious disease prophylaxis strategies were employed during this study including isolation in laminar air flow rooms, the prophylactic use of systemic antibiotics, fluconazole, acyclovir, ganciclovir, and the use of screened or filtered blood products.26 All CMV seronegative patients received either screened or filtered blood products. Acyclovir was given for prophylaxis to all herpes simplex virus seropositive patients throughout the study period. Ganciclovir was given to all CMV seropositive recipients at engraftment or at the first development of antigenemia.27-29 All patients received fluconazole.30
Causes of Death
Deaths after persistent posttransplant relapse were categorized as due to leukemia irrespective of the proximate cause. Deaths in the absence of persistent relapse were categorized as nonrelapse mortality. Infection was listed as the cause of death when a bacterial, viral, or fungal infection other than IP was the proximate cause of death in patients who had not relapsed. Infections were further categorized according to their association with or without GVHD. Deaths due to IP formed a separate category.
Statistical Analysis
The endpoints examined included survival, relapse, mortality from causes other than relapse (nonrelapse mortality), and acute GVHD. Acute GVHD was measured as grade 2 or greater during the first 100 days posttransplant or as grade 3 or 4 during the first 100 days posttransplant. Estimates of survival were obtained by the method of Kaplan and Meier31,32 where patients were censored at last follow-up if still alive. Estimates of the incidence of persistent relapse and acute GVHD were obtained using cumulative incidence estimates,33 where death without persistent relapse was regarded as a competing risk for the endpoint of persistent relapse and death without acute GVHD was regarded as a competing risk for the endpoint of acute GVHD. Patients who did not reach the appropriate endpoint or fail from the appropriate competing risk were censored on the date of latest cytogenetic evaluation for evaluating relapse and on day 100 for evaluating acute GVHD in the cumulative incidence estimates. Proportional hazards regression analysis was performed on the endpoints survival, persistent cytogenetic relapse, and acute GVHD. Variables tested for association with outcome were CSSBU categorized as less than or greater than the median, age (categorized as less than or greater than 40 years), the interval from diagnosis to transplant (categorized as less than or greater than 1 year), donor gender, and the permutations of CMV seropositivity of patient and donor. The association of these variables with the hazard appropriate for each endpoint was examined in univariable analysis, and variables that showed statistically significant or suggestive associations were examined as covariables in multivariable analysis. All P values from these models were derived from the Wald test or from likelihood ratio tests where the Wald test could not be computed, and are two-sided. No adjustments were made for multiple comparisons. The data set was locked for recording events on November 1, 1996.
RESULTS
Busulfan Levels
Table 1 presents the distribution of CSSBU for the patients in this study. The mean of these levels was 917 ng/mL (range, 642 to 1,749) and the median level was 917 ng/mL. The median clearance was 3.03 mL/min/kg, comparable to previous observations in adults.10 11
Table 2 shows the patient characteristics at the time of transplant for the groups categorized as having CSSBU below or above the median. As shown, the two groups were generally similar except for a trend towards more younger patients in the group with higher CSSBU levels.
Regimen-Related Toxicity (RRT)
The median RRT score for patients above and below the median CSSBU was 1 and there was no statistically significant difference in the distribution of RRT scores between the two groups (Mann-Whitney test, P = .477). The most common RRT was mucositis (95% of patients) followed by veno-occlusive disease of the liver (VOD) (34%). Only one patient (CSSBU 833 ng/mL) developed severe RRT (grade 3 or 4), and that patient died on day 34 of VOD.
Relapse
Seven patients (five of 39 patients transplanted in CP and two of six transplanted in AP) had reappearance and persistence of Ph chromosomes in marrow cell metaphases after the transplant on days 57, 79, 183, 211, 227, 423, and 749. All relapses occurred in patients with CSSBU below the median value.
In the group of 21 patients with CSSBU below the median who were evaluated for relapse, the median number of cytogenetic examinations before relapse was detected was four (range, three to seven), and the median number of metaphases examined in each test was 20 (range, one to 30). In the group of 23 patients with CSSBU at or above the median, the median number of cytogenetic examinations was four (range, three to nine), and the median number of metaphases examined was 20 (range, two to 30).
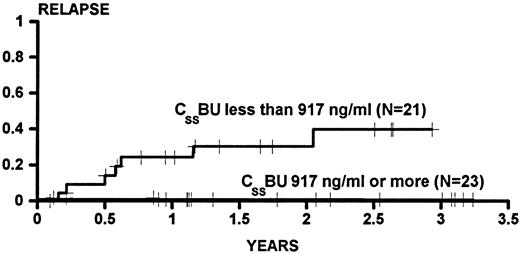
Figure 1 shows the cumulative incidence estimates of persistent relapse for patients categorized as having CSSBU below (cumulative incidence, 0.384) or above (cumulative incidence, 0.000) the median. The difference between the groups was statistically significant (P = .0009 univariate). In univariable Cox analysis, no other variable was significantly associated with relapse. However, because previous experience in larger series had demonstrated an association of relapse with donor gender and with age, the association of CSSBU with relapse was examined in multivariable analysis, and the P value was .0003 after adjustment for male donor and age greater than 40 years.
The cumulative incidence of relapse for patients transplanted for CML in CP or AP categorized on the basis of BU steady state concentrations (CSSBU) greater than or less than the median (917 ng/mL) during conditioning. Patients were censored on the date of last cytogenetic examination. One patient who died on day 34 did not have any posttransplant cytogenetic examinations and was not evaluated for relapse. Tic marks denote patients at risk of relapse. The difference between the groups is statistically significant (P = .0009).
The cumulative incidence of relapse for patients transplanted for CML in CP or AP categorized on the basis of BU steady state concentrations (CSSBU) greater than or less than the median (917 ng/mL) during conditioning. Patients were censored on the date of last cytogenetic examination. One patient who died on day 34 did not have any posttransplant cytogenetic examinations and was not evaluated for relapse. Tic marks denote patients at risk of relapse. The difference between the groups is statistically significant (P = .0009).
Graft Rejection
All patients achieved functional marrow engraftment and there were no significant differences between the groups of patients with CSSBU above or below the median for speed of engraftment as measured by the days on which granulocytes reached 100/μL (medians, 14 and 15, respectively, P = .54) or 500/μL (medians, 20 and 24, respectively, P = .52) or the days after which platelets were maintained without transfusion at 20,000/μL for 7 days (medians, 20 and 21 days, respectively, P = .26).
Tests for chimerism demonstrated persistent donor hematopoiesis in all of the patients who relapsed.
Survival
Eleven patients (nine of 39 patients transplanted during CP and two of six patients transplanted during AP) have died. Four of the deaths occurred in patients who had CSSBU above the median, and seven deaths were in patients with CSSBU below the median. Three of the CP patients died of disseminated aspergillosis on days 114, 211, and 604, three died of CMV pneumonia on days 160, 268, and 280, one died with veno-occlusive disease of the liver on day 34, one died of complications of chronic GVHD on day 215, and one died on day 360 after relapse. The deaths in patients transplanted during AP were due to posttransplant relapse and occurred on days 642 and 847 after transplant.
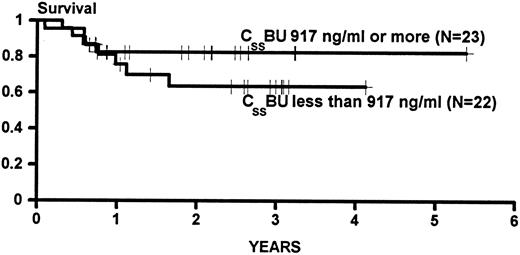
Figure 2 presents the Kaplan Meier statistics on survival for patients in this study characterized on the basis of the CSSBU being above or below the median. The difference between the two groups was not statistically significant (P = .33, power = 0.26 to detect a difference between 0.82 and 0.64 [Fig 2]).
Kaplan Meier statistics on the survival of patients transplanted for CML in CP or AP categorized on the basis of BU steady state concentrations (CSSBU) greater than or less than the median (917 ng/mL) during conditioning. Patients were censored on the date of last contact. Tic marks denote survivors. The difference between the groups is not statistically significant (P = .33)
Kaplan Meier statistics on the survival of patients transplanted for CML in CP or AP categorized on the basis of BU steady state concentrations (CSSBU) greater than or less than the median (917 ng/mL) during conditioning. Patients were censored on the date of last contact. Tic marks denote survivors. The difference between the groups is not statistically significant (P = .33)
In univariable Cox analysis, there was no statistically significant association of survival with any of the variables examined.
Nonrelapse Mortality
As described above, there were eight deaths in patients who had not relapsed. Four of the patients with CSSBU below the median died on days 34, 215, 280, and 604 of VOD, chronic GVHD, CMV pneumonia, and disseminated aspergillus infection, respectively. Two patients with CSSBU above the median died of disseminated aspergillus infection on days 114 and 211, and two died of CMV pneumonia on days 160 and 268.
The cumulative incidence of death without relapse was 0.208 in patients with CSSBU below the median and 0.176 in patients with CSSBU above the median. These incidences were not significantly different.
GVHD
Acute GVHD.One patient (CSSBU 833 ng/mL) developed grade 4 acute GVHD and died of VOD on day 34. Grade 3 acute GVHD developed in two patients in the group with CSSBU below the median and five patients with levels above the median. The cumulative incidence of grade 2 or worse acute GVHD was 0.57 in patients in the group with levels above the median and 0.54 in the group with levels below the median (P = .94).
Chronic GVHD.Of the 23 patients with CSSBU above the median, 17 (74%) developed clinically extensive chronic GVHD and three of these died (one of aspergillosis on day 211 and two of CMV pneumonia on days 160 and 268). Of the 22 patients with CSSBU below the median, 14 developed clinically extensive chronic GVHD, and three of these died (one each of aspergillosis on day 604, CMV pneumonia on day 280, and pneumonia of unknown etiology on day 215).
Status of Survivors
Thirty patients survive in continuing remission (19 from the group of 23 patients with CSSBU above the median value and 11 from the 22 patients with values below the median). There was no significant difference in the distribution of Karnofsky scores between the two groups, although two of the patients with the higher CSSBU and none of those with the lower levels had scores of less than 80. Table 3 presents the Karnofsky scores for these patients.
DISCUSSION
This study demonstrates a significantly increased risk of recurrent disease in patients with CSSBU below the median level during conditioning with the BU + CY regimen used in this study. No relapses occurred among patients who achieved BU levels above the median. The increased rate of relapse was not mediated by graft rejection, and BU levels were not significantly associated with survival, RRT, speed of engraftment, the incidence of acute or chronic GVHD, or the Karnofsky scores of disease-free survivors.
The lack of an association of CSSBU with graft rejection agrees with our previous report10 of a relationship between CSSBU and graft rejection in patients receiving grafts from HLA-identical siblings. In that study, rejection was observed only at CSSBU less than 200 ng/mL, ie, substantially lower than the lowest CSSBU observed in the present study (642 ng/mL).
The absence of severe (grade 3 or 4) RRT at CSSBU higher than 900 ng/mL contrasts with the higher incidence previously reported.10 In the previous study, severe RRT was observed in four of 11 patients with CSSBU above 900 ng/mL, whereas, in the present study no severe RRT was observed in patients with CSSBU above the median (917 ng/mL). The previous report included patients with a variety of diagnoses. In that study, only one of the patients with severe RRT had CML, and the others had diagnoses of acute myelogenous leukemia, multiple myeloma, and myelodysplasia. The relationship between RRT and BU plasma concentration reported in our previous study was in general agreement with that reported by others.11 It is likely that the ability to tolerate higher CSSBU in BU + CY regimens is diagnosis-dependent and perhaps reflects a more intensive use of prior cytotoxic therapy in patients transplanted for conditions other than CML.
We have reported elsewhere that adjustment of the oral BU dose based on first dose pharmacokinetics resulted in BU plasma levels within 10% of the target.12 Given the findings reported here, we have revised our current protocol for allogeneic marrow transplantation of patients with CML in CP by incorporating this dose-adjustment strategy to ensure that the CSSBU is at least 900 ng/mL. We are not adjusting BU doses downward because we have not observed an increased risk of severe RRT in CML patients treated with this regimen and achieving high CSSBU. Maintaining CSSBU above 900 ng/mL may result in a reduced relapse rate compared with the overall relapse rate seen in this study and previously6 in patients receiving BU + CY conditioning.
Supported by Grants No. CA 15704, CA 18029, CA 18221, and CA 09515 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
Address reprint requests to R.A. Clift, FIMLS, The Fred Hutchinson Cancer Research Center, 1124 Columbia St, Seattle, WA 98104-2092.