Abstract
Bacterial DNA and synthetic oligodeoxynucleotides containing the CpG motif (CpG ODN) can activate various immune cell subsets, including natural killer cells and macrophages. We evaluated whether the combination of CpG ODN and antitumor monoclonal antibody is effective at preventing tumor growth in an immunocompetent murine lymphoma model. CpG ODN–activated murine splenocytes induced lysis of tumor targets more effectively than unactivated splenocytes. These effector cells were also superior to unactivated splenocytes or cells activated with a control methylated ODN at inducing antibody-mediated lysis of 38C13 murine lymphoma cells. In vivo, CpG ODN alone had no effect on survival of mice inoculated with 38C13 cells. However, a single injection of CpG ODN enhanced the antitumor response to antitumor monoclonal antibody therapy. Ninety percent of mice treated with monoclonal antibody alone developed tumor compared with 20% of mice treated with antibody and CpG ODN. These antitumor effects were less pronounced when treatment consisted of an identical ODN containing methylated CpG dinucleotides. A single dose of CpG ODN appeared to be as effective as multiple doses of interleukin-2 at inhibiting tumor growth when combined with antitumor monoclonal antibody. We conclude that immunostimulatory CpG ODN can enhance antibody dependent cellular cytotoxicity and warrant further evaluation as potential immunotherapeutic reagents in cancer.
RECENT STUDIES have demonstrated that bacterial DNA, but not vertebrate DNA, can have significant immunostimulatory effects on B cells,1-3 natural killer (NK) cells,4-6 and macrophages.7 NK cells can be activated by the nucleic acid fraction of attenuated mycobacterial cells (Bacillus-Calmette-Guerin [BCG])8 and by DNA synthesized in 30mer and 45mer lengths from the BCG genome. Such activated NK cells produce interferon-γ (IFN-γ) and show enhanced NK cytolytic activity.6 NK cells activated by bacterial DNA have in vivo antitumor activity as well.8 Recent studies demonstrate that macrophages are activated by the bacterial DNA that is internalized after phagocytosis.7 These immunostimulatory effects are due, at least in part, to sequences containing unmethylated CpG dinucleotides, because the effects are abolished by cytosine methylation.3 In addition, synthetic oligodeoxynucleotides containing CpG motifs (CpG ODN) also can enhance NK and macrophage activity.4,7 Vertebrate DNA, but not bacterial DNA, exhibits CpG suppression and extensive methylation of cytosines in CpG dinucleotides. We therefore have postulated that lymphocyte activation by unmethylated CpG dinucleotides may have evolved as a mechanism by which the immune response to microbial infection could be enhanced.3
The immune response normally involves the integrated production of a variety of cytokines that work in concert both locally and systemically. At the cellular level, CpG ODN induce secretion of interleukin-6 (IL-6), IL-12, and IFN-γ2,4,9 but not IL-2. In unfractionated spleen cultures, NK activation by CpG ODN is unaffected by removal of B cells or T cells, but decreases markedly upon neutralization of IL-12, tumor necrosis factor-α (TNF-α), and IFN-γ.4 Interestingly, highly purified NK cells are not activated by CpG ODN.4 9 It therefore appears that stimulation of an additional population of cells, such as monocytes/macrophages, by CpG ODN is important in achieving NK activation.
The mechanisms responsible for CpG ODN–induced immune activation are under intensive investigation. Stacey et al7 have found that bacterial DNA and CpG ODN can activate transcription factor nuclear factor-κB, which subsequently leads to induction of other genes involved in inflammation.7 Preliminary data from our laboratory also suggest that DNA containing the CpG dinucleotide functions by inducing generation of reactive oxygen species.10
Both NK cells and monocytes/macrophages participate in antibody-dependent cellular cytotoxicity (ADCC). Activation of NK cells with cytokines can enhance ADCC in vitro and the efficacy of monoclonal antibody (MoAb) therapy in vivo.11 Enhancement of tumor immunity may be most effective if agents are used that can orchestrate the immune response, including cytokine production and cellular activation, in a manner that reflects physiological responses. CpG ODN are such agents. The current studies were designed to evaluate whether CpG ODN can enhance ADCC in vitro and improve the therapeutic efficacy of antitumor MoAb therapy in vivo.
MATERIALS AND METHODS
Tumor model.The 38C13 murine B-cell lymphoma model has been used extensively in studies of antitumor MoAb therapy.11-14 38C13 cells were grown in RPMI 1640 supplemented with 100 U/mL of penicillin and streptomycin, 2-mercaptoethanol, and 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) that was heat inactivated at 56°C (complete media). Cells were used in log-phase growth. The idiotype expressed by the surface IgM of 38C13 serves as a highly specific tumor antigen. The IgG2a anti-idiotype MoAb designated MS11G6 has been previously described14 15 and is referred to as antitumor MoAb. It was purified from cell culture supernatant by affinity chromatography using protein A and used in both the in vitro and in vivo assays.
Oligodeoxynucleotide preparation.Phosphorothioate oligodeoxynucleotides were kindly provided by Genta (San Diego, CA) or purchased from Oligos etc (Wilsonville, OR). An immunostimulatory CpG oligodeoxynucleotide (with the sequence 5′ TCT CCC AGC GTG CGC CAT 3′) was selected for further study when it was found to have a significant effect on murine NK cells with little effect on murine B cells. This CpG oligodeoxynucleotide (henceforth referred to as CpG ODN) at concentrations ranging from 1.25 to 20 μg/mL also had no effect on the in vitro proliferation of 38C13 cells (data not shown). It should be noted that this sequence is also antisense to the human Bcl-2 gene and has partial homology to the antisense to mouse Bcl-2 gene. The control oligodeoxynucleotide (control methylated ODN) had an identical sequence, but contained methylcytosines in the CpG motifs (see Fig 1). Lipopolysaccharide levels were assessed by the Limulus assay and were always less than 12.5 ng/mg of ODN.
Sequences of CpG ODN and methylated control ODN. Underlined cytosines in the control ODN were methylated.
Sequences of CpG ODN and methylated control ODN. Underlined cytosines in the control ODN were methylated.
In vitro chromium release assay.A standard chromium release assay was used to detect cytotoxicity. Spleens of naive 6- to 8-week-old C3H/HeN mice were homogenized, and erythrocytes were removed by hypo-osmolar lysis with a solution containing 0.15 mol/L NH4Cl, 1.0 mmol/L KHCO3 , 0.1 mmol/L Na2EDTA at a pH of 7.4. B cells were removed by anti-Ig selection using a biotinylated goat antimouse Ig (Sigma, St Louis, MO) and avidin-coated micromagnetic beads eluted over a magnetic column (Miltenyi Biotech, Auburn, CA). The eluted cells were washed with media and cultured in complete media alone or media supplemented with CpG ODN, control methylated ODN, or 500 U/mL of recombinant human IL-2 (Chiron, Emeryville, CA) for 24 to 72 hours before use as effector cells. Target cells (38C13) were labeled with 200 μCi of Cr51 (Amersham Life Sciences, Arlington Heights, IL) for 1 hour and washed three times. Cells were divided and incubated at 4°C for 30 minutes in complete media or media supplemented with 5 μg/mL antitumor MoAb before distribution into 96-well V-bottom culture plates (Costar, Cambridge, MA) at the desired cellular concentrations. Effector cells were added at the desired effector to target ratios. Plates were incubated for 4 hours at 37°C in a humidified environment containing 5% CO2 and supernatants evaluated by γ counting. Nonspecific release of Cr51 (percent release from target cells incubated for 4 hours without effector cells or MoAb) was less than 20% for all experiments. Samples were run in triplicate, and the percentage of lysis was determined.
In vivo tumor therapy.38C13 cells grow rapidly and consistently in syngeneic, immunocompetent C3H/HeN mice. Female C3H/HeN mice, obtained from Harlan-Sprague-Dawley (Indianapolis, IN) and housed in the Animal Care Unit at the University of Iowa, were used at 6 to 8 weeks of age. Mice were inoculated with 2,500 38C13 tumor cells intraperitoneally (IP), and therapy with CpG or control methylated ODN, IL-2, and antitumor MoAb was administered IP beginning 2 days later as indicated. CpG ODN and control methylated ODN were used at a dose of 300 μg per mouse because pilot studies demonstrated systemic effects at that dose. Survival was determined, and significance with respect to time to death was assessed using Cox regression analysis.16 Mice were observed daily for signs of toxicity including level of activity, ruffled fur, diarrhea, and general appearance.
RESULTS
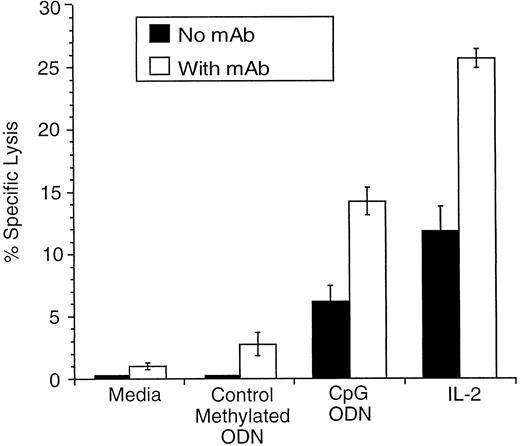
CpG ODN enhances NK cytolytic activity and ADCC in vitro.We first assessed the NK cytolytic activity of CpG ODN–stimulated effector cells in vitro using NK-sensitive YAC-1 cells as targets as previously reported.4 Effector cells stimulated with IL-2 served as a control. Unstimulated effector cells and effector cells stimulated with 2.5 μg/mL of control methylated ODN induced minimal lysis, whereas significant lysis was mediated by effector cells stimulated with 2.5 μg/mL of CpG ODN or IL-2 at a concentration of 500 U/mL (data not shown). We then explored whether CpG ODN enhances ADCC in vitro. As illustrated in Fig 2, lysis of 38C13 cells was minimal when unstimulated effector cells or effector cells stimulated with control methylated ODN were used. IL-2–activated effector cells were capable of mediating lysis. This was enhanced further when antitumor MoAb was added. Similar, although less profound effects were noted when CpG ODN-stimulated effector cells were used. Thus, CpG ODN enhanced ADCC mediated by antitumor MoAb, but not to the same degree as IL-2.
CpG ODN or IL-2 prestimulation of mononuclear splenocytes in combination with MoAb enhances lysis of 38C13 cells in vitro. Splenocytes were activated as described in the Materials and Methods. Target 38C13 cells were labeled with chromium-51 for 1 hour followed by incubation with MoAb (5 μg/mL) for 30 minutes at 4°C. Effector cells and target cells were plated at a 25:1 effector-target ratio and chromium release was determined.
CpG ODN or IL-2 prestimulation of mononuclear splenocytes in combination with MoAb enhances lysis of 38C13 cells in vitro. Splenocytes were activated as described in the Materials and Methods. Target 38C13 cells were labeled with chromium-51 for 1 hour followed by incubation with MoAb (5 μg/mL) for 30 minutes at 4°C. Effector cells and target cells were plated at a 25:1 effector-target ratio and chromium release was determined.
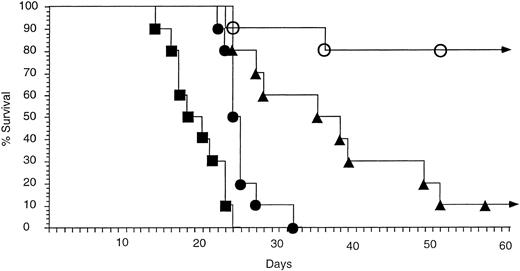
CpG ODN enhances antitumor therapy with antitumor MoAb.Previous studies have demonstrated that antitumor MoAb in the 38C13 model has a moderate antitumor effect when administered in large doses (100 μg) 4 hours after inoculation of the mice with tumor. The antitumor effect of MoAb is limited in this rapidly growing tumor model when therapy is delayed for greater than 24 to 48 hours after tumor inoculation.14,15 17 We therefore assessed whether treatment with CpG ODN allows for more effective MoAb therapy when treatment with MoAb is delayed beyond the time when MoAb is usually effective (Fig 3). Mice were inoculated with 2,500 38C13 cells and divided into groups of 10. Mice received no therapy (group 1), treatment with a single IP injection of 300 μg CpG ODN on day 2 (group 2), therapy with a single injection of 50 μg antitumor MoAb IP on day 3 (group 3), or both CpG ODN and antitumor MoAb (group 4). No toxicity was noted in any group. All mice in the control group (group 1) died within 24 days. Mice that received CpG ODN alone (group 2) had slightly improved survival, but all developed lymphoma and died within 34 days. Mice treated with antitumor MoAb alone had prolonged survival when compared with untreated mice, but only 1 of 10 mice achieved long-term survival. In contrast, 8 of 10 mice treated with CpG ODN followed by antitumor MoAb survived without evidence of tumor. The survival of this group was highly significant when compared with the other groups (v CpG ODN alone, P < .001; v MoAb alone, P = .0109).
CpG ODN and MoAb are more effective than MoAb alone in vivo at preventing death from tumor. Immunocompetent C3H mice were inoculated with 2,500 tumor cells IP on day 0. Therapy consisted of (▪) no ODN or MoAb, (▴) MoAb alone, (•) CpG ODN alone, or (○) CpG ODN and MoAb. CpG ODN (300 μg) was administered IP on day 2, and MoAb (50 μg) was administered on day 3. Each group contained 10 mice. Curves depict percent survival over 60 days. No mice living at day 60 developed tumor, and all survived more than 5 months. No toxicity was observed in any group.
CpG ODN and MoAb are more effective than MoAb alone in vivo at preventing death from tumor. Immunocompetent C3H mice were inoculated with 2,500 tumor cells IP on day 0. Therapy consisted of (▪) no ODN or MoAb, (▴) MoAb alone, (•) CpG ODN alone, or (○) CpG ODN and MoAb. CpG ODN (300 μg) was administered IP on day 2, and MoAb (50 μg) was administered on day 3. Each group contained 10 mice. Curves depict percent survival over 60 days. No mice living at day 60 developed tumor, and all survived more than 5 months. No toxicity was observed in any group.
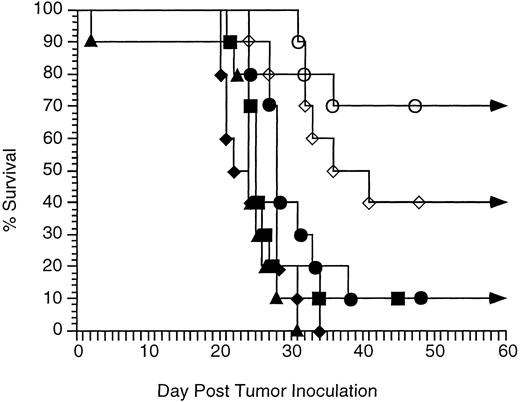
Comparison of antitumor therapy with CpG ODN and antitumor MoAb to therapy with IL-2 and antitumor MoAb.IL-2 has been shown to enhance the efficacy of anti-idiotype MoAb therapy in the 38C13 model. IL-2 is most effective when administered repeatedly.11 We therefore compared in vivo therapy of antitumor MoAb with CpG ODN to therapy with MoAb and IL-2 (Fig 4). Mice in the IL-2 groups received 50,000 U IL-2 twice daily for 3 days. Mice received no therapy (group 1), treatment with a single IP injection of 300 μg CpG ODN on day 2 (group 2), therapy with a single injection of 50 μg antitumor MoAb IP on day 3 (group 3), both CpG ODN and antitumor MoAb (group 4), IL-2 alone (group 5), or IL-2 and MoAb (group 6). CpG DNA plus antitumor MoAb therapy resulted in long-term survival of 70%, whereas IL-2 plus antitumor MoAb resulted in long-term survival of only 40%. This difference did not reach statistical significance (P = .20).
Therapy with CpG ODN and MoAb enhances survival as effectively as therapy with IL-2 and MoAb. Immunocompetent C3H mice were inoculated with 2,500 tumor cells IP on day 0. Therapy consisted of MoAb alone, CpG ODN alone, IL-2 alone, IL-2 and MoAb, or CpG ODN and MoAb. IL-2 (50,000 U) was administered IP twice daily on days 2, 3, and 4. CpG ODN (300 μg) was administered IP on day 2, and MoAb (50 μg) was administered on day 3 as in Fig 3. Each group contained 10 mice. Curves depict the percentage of survival over 60 days. No mice living at day 60 developed tumor, and all survived more than 5 months. No toxicity was observed in any group. (▪) No therapy; (•) MoAb on day 3; (▴) CpG ODN on day 2; (♦) IL-2 on days 2, 3, and 4; (⋄) IL-2 on days 2, 3, and 4 and MoAb on day 3; (○) CpG ODN on day 2 and MoAb on day 3.
Therapy with CpG ODN and MoAb enhances survival as effectively as therapy with IL-2 and MoAb. Immunocompetent C3H mice were inoculated with 2,500 tumor cells IP on day 0. Therapy consisted of MoAb alone, CpG ODN alone, IL-2 alone, IL-2 and MoAb, or CpG ODN and MoAb. IL-2 (50,000 U) was administered IP twice daily on days 2, 3, and 4. CpG ODN (300 μg) was administered IP on day 2, and MoAb (50 μg) was administered on day 3 as in Fig 3. Each group contained 10 mice. Curves depict the percentage of survival over 60 days. No mice living at day 60 developed tumor, and all survived more than 5 months. No toxicity was observed in any group. (▪) No therapy; (•) MoAb on day 3; (▴) CpG ODN on day 2; (♦) IL-2 on days 2, 3, and 4; (⋄) IL-2 on days 2, 3, and 4 and MoAb on day 3; (○) CpG ODN on day 2 and MoAb on day 3.
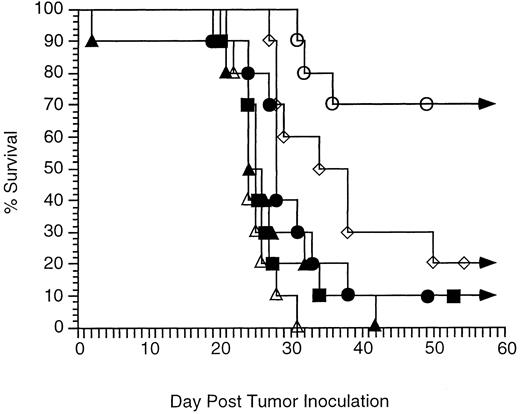
Comparison of antitumor therapy with CpG ODN and antitumor MoAb to therapy with control methylated ODN and antitumor MoAb.In vitro studies suggested the control methylated ODN had little effect on enhancement of ADCC. However, it was possible other mechanisms, such as antisense activity, contributed to the antitumor effect seen in vivo with CpG ODN and MoAb. We therefore evaluated therapy in vivo with MoAb and control methylated ODN in parallel with the IL-2 study outlined above. Control methylated ODN added little to the therapeutic efficacy of MoAb alone (control methylated ODN and MoAb v MoAb alone, P = .12) (Fig 5) in contrast to the studies outlined in Fig 3 where therapy with MoAb and CpG ODN was more effective than therapy with MoAb alone.
Therapy with CpG ODN and MoAb enhances survival more effectively than therapy with control methylated ODN and MoAb. Immunocompetent C3H mice were inoculated with 2,500 tumor cells IP on day 0. Therapy consisted of MoAb alone, control methylated ODN alone, CpG ODN alone, control methylated ODN and MoAb, or CpG ODN and MoAb. Mice were followed for tumor development, toxicity, and survival. Each group contained 10 mice. Curves depict percent survival over 60 days. No mice living at day 60 developed tumor, and all survived more than 5 months. No toxicity was observed in any group. (▪) No therapy; (•) MoAb on day 3; (▴) control methylated ODN on day 2; (▵) CpG ODN on day 2; (⋄) control methylated ODN on day 2 and MoAb on day 3; (○) CpG ODN on day 2 and MoAb on day 3.
Therapy with CpG ODN and MoAb enhances survival more effectively than therapy with control methylated ODN and MoAb. Immunocompetent C3H mice were inoculated with 2,500 tumor cells IP on day 0. Therapy consisted of MoAb alone, control methylated ODN alone, CpG ODN alone, control methylated ODN and MoAb, or CpG ODN and MoAb. Mice were followed for tumor development, toxicity, and survival. Each group contained 10 mice. Curves depict percent survival over 60 days. No mice living at day 60 developed tumor, and all survived more than 5 months. No toxicity was observed in any group. (▪) No therapy; (•) MoAb on day 3; (▴) control methylated ODN on day 2; (▵) CpG ODN on day 2; (⋄) control methylated ODN on day 2 and MoAb on day 3; (○) CpG ODN on day 2 and MoAb on day 3.
DISCUSSION
B-cell lymphomas are among the most sensitive tumors to MoAb-based immunotherapy. Agents known to enhance NK activity, such as IL-2, have been shown to enhance the antitumor effects of antilymphoma MoAb. We used a well-established lymphoma model to evaluate whether the antitumor effects of MoAb can be enhanced by CpG ODN that induce NK activation. There was clear synergy between CpG ODN and antitumor MoAb in this model and the most likely explanation for this finding is enhanced ADCC. It is unlikely that the CpG ODN has a direct effect on tumor cells, given tumor proliferation was not inhibited in vitro by CpG ODN and only minimal therapeutic benefit was seen in the group treated with CpG ODN alone. An alternative explanation is that the CpG ODN-induced production of cytokines rendered the tumor cells more sensitive to ADCC.
These effects were largely eliminated when an ODN containing identical sequences but having methylated cytosines in the CpG motif was used. Because the 5-methyl cytosine base substitutions actually increase the affinity for hybridization to complementary mRNA, this finding demonstrates that the antitumor effect of this ODN cannot be attributed solely to an antisense mechanism.
Anecdotal reports of tumor regression after systemic bacterial infection have been observed for centuries. Experimental antitumor therapy with heat-inactivated bacteria was reported by Dr William Coley18 in the 1890s. Dr Coley's original attempt to use bacteria as an antitumor agent involved the use of live cultures of streptococci.18 This resulted in tumor regression, but proved to be toxic with the first patient almost dying of erysipelas. Subsequent studies by Coley involved a mixture of heat-killed Streptococci and Serratia (then known as Bacillus prodigiosus).19 It was this preparation that is now known as Coley's toxin. Much of the antitumor activity of Coley's toxin is currently attributed to endotoxin. A number of cytokines induced by endotoxin (such as TNF-α and IFN-γ) have been produced in recombinant form and have been shown to have antitumor activity.20 However, it is curious to note that Coley's original success was with an organism that does not produce endotoxin. It is possible bacterial DNA with its unmethylated CpG motifs played a role in the antitumor effects seen in Coley's original preparation. Whether the responses seen by Dr Coley were related to the immunostimulatory effects of streptococcal DNA, the data presented above indicate that motifs found in bacterial DNA can have antitumor effects, particularly when used with other agents such as MoAb.
A number of important questions remain to be answered. The mechanisms by which bacterial DNA and CpG ODN interact with the cell surface of immune effector cells are internalized and induce activation signals that lead to NK cell and macrophage stimulation need to be clarified. It will also be important to understand more thoroughly how different subsets of immune cells respond to CpG ODN and what aspects of that response are related directly to CpG ODN stimulation or indirectly to cytokines such as IL-6, IL-12, or IFN. Although we observed no toxicity in vivo, the toxicity profile of CpG ODN will require definition. We detected no direct effect of the CpG ODN on 38C13 lymphoma cells; however, it is possible the CpG ODN induced changes in the tumor cells that rendered them more sensitive to MoAb therapy. These studies therefore need to be confirmed in another tumor model and using other CpG ODN.
As we learn more about the immune system, it is becoming obvious that an effective antitumor immunotherapy will need to integrate several aspects of the immune system, including establishment of appropriate cytokine profiles, modification of tumor immunogenicity, enhancement of tumor antigen recognition, and appropriate effector cell expansion and activation. The studies outlined above demonstrate immunostimulatory DNA in general, and CpG ODN in particular, may have a role to play in the development of such a therapy.
ACKNOWLEDGMENT
We thank Yina Hsing and Wendy Rasmussen for technical assistance and Charles Davis for assistance with statistical analysis.
Supported in part by a grant from the University of Iowa Cancer Center. J.E.W. is supported by National Institutes of Health Grant No. T32HL07344, and A.M.K. is supported by a career development award from the Department of Veterans Affairs.
Address reprint requests to George J. Weiner, MD, C32K GH, The University of Iowa Hospitals and Clinics, 200 Hawkins Dr, Iowa City, IA 52242.