Abstract
Mice harboring a mutation in the microphthalmia (mi ) gene display a variety of abnormalities, including microphthalmia, depletion of skin melanocytes, deafness, a defect in osteoclasts, and a major decrease in mast cell number and function. However, despite the possible critical role played by this protein in mast cell development and function, characterization of its mRNA and protein synthesis in these cells has not yet been performed. In this study, we investigated the regulation of the synthesis of mi in murine mast cells activated by various physiologic stimuli. Using a specific rabbit polyclonal anti-mi antibody, we found that interleukin-3, interleukin-4, or aggregation of the mast cell high-affinity receptor for IgE (FcεRI) induced the synthesis of mi protein in these cells. None of these stimuli significantly affected the level of mi mRNA in the mast cells at any of the time points tested. Also, using this specific anti-mi antibody, an increase in mi protein synthesis was shown during differentiation of mast cells from their bone marrow cell precursors. Moreover, a complex containing mi bound to upstream stimulating factor 2 was detected only in activated mast cells. We conclude that the regulation of mi expression is on the translational level. Thus, stimulation of mast cells by a variety of stimuli elicits a signaling pathway that regulates mi expression.
ALTHOUGH THE COMPLEX mechanisms that are involved in the regulation of proliferation, differentiation, and tissue-specific gene expression in mast cells are only beginning to be explored, it is clear that coordinated transcriptional regulation plays a pivotal role in all of these functions. Transcription factors with a limited tissue-specific distibution were shown to play a crucial role in the differentiation of several cell types, such as MyoD in myocytes.1 In mast cells, the only known transcription factor that plays a major role in the regulation of growth and differentiation is the microphthalmia (mi) protein.2 Mutations in the gene encoding for this transcription factor were shown to cause the mouse microphthalmia phenotype.3,4 The mouse microphthalmia phenotype is now known to contain more than 10 allelic variations.4,5 Homozygous mutations at this locus cause small unpigmented eyes, skin and inner ear melanocyte depletion, microphthalmia, deafness, a defect in osteoclasts, and a major decrease in mast cell number.5 Jippo-Kanemoto et al6,7 showed that, although mi/mi mice are mast cell deficient, splenic cells from these mice develop into mast cells when cultured with conditioned medium. However, these cells are different from mast cells grown from normal mice in many important aspects. For example, when mast cells from mi/mi mice are cocultured with fibroblasts, they fail to enter the S phase and gradually disappear from the tissue culture plates.6 Furthermore, they express only low levels of c-Kit and nerve growth factor 140-kD receptors, two growth factor receptors with important roles in mast cell development.7 8
The isolation of the gene causing the mi mutation was recently achieved by two groups as a result of the fortunate observation of homozygous transgenic mice strains displaying similar phenotypes to that of the mi/mi mice.3 4 The integration site of the transgene from these mice was then cloned and analyzed. Computer analysis of the nucleotide sequence showed that the mi gene encodes a novel member of the basic helix-loop-helix leucine zipper (bHLH-ZIP) protein family of transcription factors. Northern blot of mRNA from different tissues and cell types showed that mi mRNA is present only in a limited number of tissues and cell types (mast cells, melanocytes, and the heart).
Because mi was shown to have an essential role in mast cell function and development, we decided to characterize the expression patterns of this transcription factor in mast cells. We developed a specific rabbit polyclonal anti-mi antibody that enabled us to perform a study on the regulation of mi expression in mast cells. In this report, we describe the induction of mi protein in murine mast cells by various stimuli. The mast cell mi mRNA was further characterized and was found to have the same transcriptional start site as that of melanocytes.
MATERIALS AND METHODS
Cell culture and tissues.MC-9 cells1 obtained from the American Type Culture Collection (Rockville, MD) were maintained at 37°C in RPMI-1640 medium supplemented with 2 mmol/L L-glutamine, 2 mmol/L nonessential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin (GIBCO, Grand Island, NY), 50 μmol/L β-mercaptoethanol (Fisher Scientific, Medford, MA), 10% fetal calf serum (Bio-Lab, Jerusalem, Israel; growth medium), and the lymphokines interleukin-3 (IL-3) and IL-4 (kindly provided by I. Clark-Lewis, Vancouver, British Columbia, Canada). Bone marrow-derived mast cells (BMMC) were developed in culture from BM cells derived from C57BL mice10 and grown and maintained at 37°C in growth medium supplemented with IL-3 and IL-4. Melanocytes (B16S10.911) were maintained at 37°C in growth medium. Heart, liver, and spleen were obtained from 2-month-old C57BL male mice.
Growth factor-mediated activation.A suspension of 2.5 to 5 × 106 MC-9 cells was washed and incubated with RPMI 1640 serum-free medium for 4 hours at 37°C. The cells were then washed again and incubated in RPMI 1640 medium containing 100 U of either IL-3 or IL-4 for various periods of time. The induction reaction was stopped by centrifugation. Trypan blue exclusion showed greater than 95% cell viability after each experiment.
IgE-Ag–mediated activation.IgE sensitization was performed by incubating replicates of 2.5 × 106 cells with 2.5 μg of monoclonal IgE antibodies against dinitrophenol (DNP; kindly provided by Dr F.-T. Liu, La Jolla, CA) for 45 minutes at 37°C in 200 μL of modified Tyrode's solution containing 1.0 mmol/L Ca2+, 0.3 mmol/L Mg2+, and 1 mg/mL gelatin (TG). The cells were then incubated at 37°C with 250 ng DNP coupled to bovine serum albumin (DNP-BSA) for defined periods. The induction reaction was stopped by centrifugation. Trypan blue exclusion showed greater than 95% cell viability after each experiment.
Isolation of RNA.Ten million MC-9 cells or a C57BL mouse heart were homogenized in RNAzol B (Biotecx Lab, Houston, TX) and RNA was extracted according to the manufacturer's instructions.
Northern blot analysis.12Samples of 5 to 10 μg RNA derived from MC-9 cells were concentrated and denatured in a 1:3 mixture of formaldehyde (BDH Ltd, Poole, UK) and formamide (Fluka, Buchs, Switzerland) for 10 minutes at 65°C. They were then separated on an agarose/MOPS gel (Sigma, St Louis, MO) that was blotted onto a NY 13N Nytran (Amersham, Arlington Heights, IL) in 10× SSC for 48 hours and then exposed to UV light for 4 minutes. After 2 to 3 hours of prehybridization at 42°C in a solution of 6× SSPE, 50% formamide, 1% sodium dodecyl sulfate (SDS), 5× Denhart's solution, and 100 μg/mL salmon sperm DNA, hybridization was performed in the same solution at 42°C for 24 hours using 20 ng of heat-denatured [32P]-labeled DNA probe. After an initial wash in 02× SSC, 0.1% SDS at room temperature followed by 25 minutes at 56°C, the filter was exposed to either film (Kodak Curix RP2; Eastman Kodak, Rochester, NY) for up to 2 days at −70°C or to phosphoroimaging with a Fujix Bas2000 (Fuji Photo Film Co, Tokyo, Japan) bioimage analyzer. A light-scanning densitometer was used to quantify the relative intensities of the bands on the autoradiograms.
Primer extension experiments.12An oligonucleotide 5′ GTT TTC CAG GTG GGT CTG CAG 3′ from the putative common part of the heart and melanocyte mi mRNA13 was end-labeled with polynucleotide kinase. It was then precipitated overnight with 20 to 40 μg of mRNA. The precipitate was then dissolved in a solution containing 1:5 [vol/vol] × 5 avianmyeloblastosis reverse transcriptase (AMV-RT) reaction buffer (Promega, Madison, WI), 0.25 mmol/L dNTP; heated for 3.5 minutes at 85°C; cooled at a rate of 1°C per 3 seconds to 39°C; and then 20 U of AMV-RT (Promega) and 5 U of RNAase inhibitor were added to give a 20 μL final volume. The samples were then kept at 39°C for 1 hour followed by the addition of 20 μL of 3:1 formaldehyde:formamide solution. The samples were then heated for 4 minutes at 98°C, moved immediately to ice, and loaded together with a puc18/Msp I-restricted [32P]-labeled size marker on a 4% acrylamide, 7 mol/L urea denaturing gel.
DNA probes.The mi probe was a 1.5-kb EcoRI fragment from plasmid phMI-9 that contains human melanocyte mi cDNA that is highly homologous (nearly 90% at the nucleotide level) to the mouse mi cDNA14,15 and was generously provided to us by S. Shibahara (Tohohuko University School of Medicine, Sendai, Japan). A c-fos–specific probe was used to determine the level of mRNA by Northern blot analysis. A 3.2-kb EcoRI-BamHI fragment isolated from a λ cfos (mouse)-2 clone16 and a 0.97-kb fragment bearing the rpL30 processed gene17 were used as controls for loading an equivalent amount of total RNA. The DNA fragments were labeled with [32P]-dCTP (Amersham) using the random primed labeling technique18 up to a specific activity of 3 × 108 cpm/μg. Labeled probes were used at a final concentration of 5 × 106 cpm/mL hybridization mixture.
Preparation of rabbit antimouse mi antibody.A synthetic 20 amino acid peptide, EEQSRAESECPGMNTHSRAS, corresponding to the amino acid sequence number 133 to 152 according to Hodgkinson et al3 of the open reading frame of the cloned mouse mi, was synthesized as an 8-branched peptide on multiple antigen peptide resin (ABI, Foster City, CA) and coupled to glutaraldehyde, which was used as a carrier. A search of the SwissProt Data-Bank for mi peptide homology did not show a protein with greater than 20% homology to this peptide. Primary immunization of rabbits was with 1 mg peptide mixed with RIBI adjuvant system (RIBI ImmunoChem Research, Inc, Hamilton MT, WA) administered intramuscularly. Subsequently, rabbits received two booster immunizations subcutaneously at 14-day intervals of the same amount of peptide. Seven days after the last booster, animals were bled and the presence of antibody to mi was determined.
Serum antibodies to the synthetic peptide were assayed by enzyme-linked immunosorbent assay using 2 mg of peptide and 2 mg of BSA as control per well. Both preimmune and immune sera were diluted by serial 10-fold dilutions to a final concentration of 10−4. The end point titer was defined as the immune serum dilution that gave values of absorbance of at least 0.2; these values were 10 times higher than the mean background absorbance for BSA. Detection of bound antibody was by using antirabbit IgG coupled with alkaline phosphatase and 1 mg/mL p-nitrophenyl phosphate as substrate for colorimetric detection at 405 nm.
Immunoprecipitation.Cells, which were either sensitized or not with IgE, were washed once with Dulbecco's modified Eagle's medium (DMEM) methionine-free medium (Biological Industry, Beth Haemek, Israel), resuspended in DMEM methionine-free medium containing 50 μCi/mL [35S]-methionine (Amersham), and incubated for 1 hour at 37°C. The cells were then activated with DNP-BSA, IL-3, or IL-4 for various periods. The immunoprecipitation procedure previously described19 was modified as follows. Cells (2.5 × 106) labeled with [35S]-methionine were lysed by the addition of 500 μL cold lysis buffer (0.01 mol/L Tris-HCl, pH 7.4., 1% deoxycholate, 1% Triton X-100, 0.1% SDS, 0.15 mol/L NaCl, and 0.25 mmol/L phenylmethylsulfonyl fluoride). These cells were then homogenized and the supernatants were collected after 30 minutes of spinning in a microcentrifuge at 4°C. To normalize samples before immunoprecipitation, 2 μL of each sample was removed, the [35S]-labeled proteins were trichloroacetic acid (TCA)-precipitated, and the radioactivity was determined. Equal counts from each mixture were then added to the antimouse upstream stimulating factor 2 (USF2)20 or antimouse mi antibody. The antimouse mi antibody was preincubated with or without mi peptide. After overnight incubation at 4°C, 10 mg of protein-A sepharose beads (Sigma) was added to the incubated mixtures and agitated for 3 hours at 4°C. The protein A-sepharose beads were washed three times with lysis buffer and then washed again with Tris-EDTA buffer.
In a few of the experiments in which the cells were activated by IL-3, the immunoprecipitation was performed with rabbit antimyosin antibody (kindly provided by Dr S. Ravid, Hebrew University of Jerusalem, Jerusalem, Israel) raised against nonmuscle myosin II from Dictyostelium, which also cross-reacts with myosin II from several mammalian cells.
Coimmunoprecipitation.19,21The immunocomplexes derived from the treatment of the lysates with either USF2 antibody or mi antibody were dissociated by boiling for 10 minutes in the presence of 0.5% SDS and then diluted fivefold with the same buffer without SDS. The samples were then reprecipitated on protein A beads with the second antibody (anti-USF2, anti-mi, or anti-myosin antibody). The precipitates were separated by discontinuous 14% acrylamide-bisacrylamide SDS slab gels, along with molecular weight markers. Gels were dried and exposed to Kodak X-Omat AR film at room temperature for various periods. Quantitation of the autoradiographic bands was performed by densitometric analysis.
RESULTS
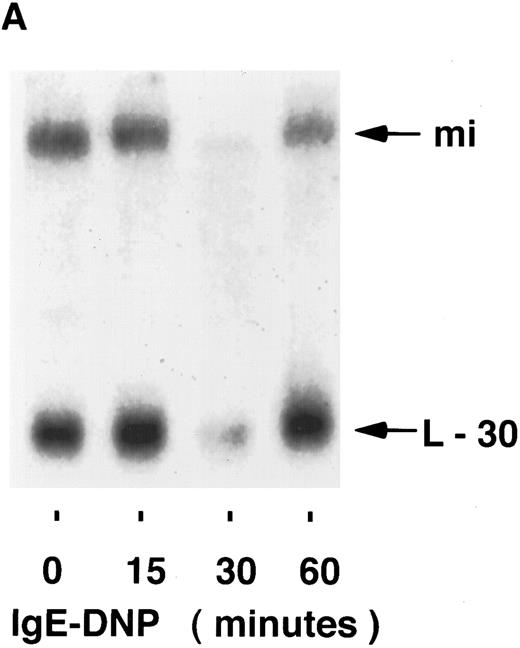
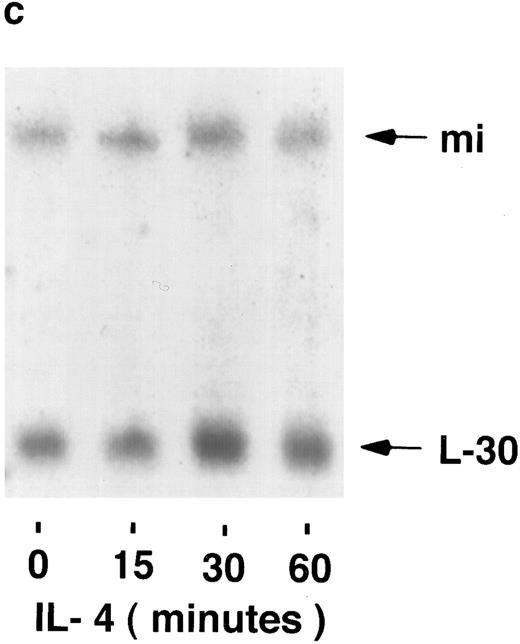
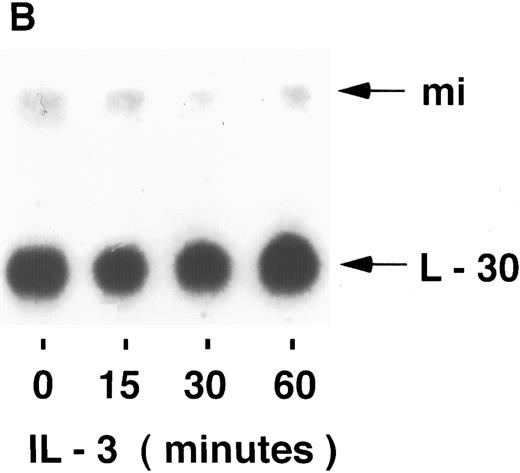
mi mRNA in mast cells.To examine the possibility that the regulation of mi expression is at the level of mRNA accumulation, the stimuli-dependent induction of mi mRNA was determined by Northern blot analysis. Using the human mi cDNA as a probe, mi mRNA in MC-9 cells was found to be slightly larger than 5 kb, as found previously.3 The possible induction of mi mRNA accumulation was then determined in MC-9 cells stimulated by IgE-Ag, IL-3, or IL-4. In several experiments, none of the stimuli mentioned above significantly affected the steady state level of mi mRNA in these cells at any of the time points tested in the range of 15 to 60 minutes (Fig 1). The intensities of the mi and L-30 bands were quantified and their ratios were determined for each time point. No significant changes were observed in these ratios at the various time points, as determined by densitometric analysis. Using the same blots, an induction in c-fos mRNA accumulation was observed in cells stimulated either by IL-3 (up to 5-fold ± 0.5 [standard error (SE)], n = 3, of the control values) or by IgE-Ag (up to 10-fold + 1 [SE], n = 3 of the control values; Fig 2) but not in those stimulated by IL-4 (data not shown). We have previously shown that IL-4 does not induce c-fos mRNA in these cells.22
Kinetics of mi mRNA accumulation in activated mast cells. Cytoplasmic RNA was extracted from IgE-Ag–stimulated (A), IL-3–stimulated (B), or IL-4–stimulated (C) MC-9 cells, and Northern blot analysis was performed as described in Materials and Methods. One representative experiment of three performed is shown.
Kinetics of mi mRNA accumulation in activated mast cells. Cytoplasmic RNA was extracted from IgE-Ag–stimulated (A), IL-3–stimulated (B), or IL-4–stimulated (C) MC-9 cells, and Northern blot analysis was performed as described in Materials and Methods. One representative experiment of three performed is shown.
Kinetics of c-fos mRNA accumulation in activated mast cells (IL-3 [A] and IgE-DNP [B]). Northern blot analysis was performed as described in Fig 1. One representative experiment of two performed is shown.
Kinetics of c-fos mRNA accumulation in activated mast cells (IL-3 [A] and IgE-DNP [B]). Northern blot analysis was performed as described in Fig 1. One representative experiment of two performed is shown.
mi mRNA starting site (primer extension experiments) in mast cells. An oligonucleotide 5′ GTT TTC CAG GTG GGT CTG CAG 3′ from the putative common part of the heart and melanocyte mi mRNA was end-labeled and then the mRNA was precipitated and reverse transcribed as described in Materials and Methods. The samples were then loaded together with a puc18/Msp I-restricted [32P]-labeled size marker on a 4% acrylamide 7 mol/L urea denaturing gel. One representative experiment of two performed is shown.
mi mRNA starting site (primer extension experiments) in mast cells. An oligonucleotide 5′ GTT TTC CAG GTG GGT CTG CAG 3′ from the putative common part of the heart and melanocyte mi mRNA was end-labeled and then the mRNA was precipitated and reverse transcribed as described in Materials and Methods. The samples were then loaded together with a puc18/Msp I-restricted [32P]-labeled size marker on a 4% acrylamide 7 mol/L urea denaturing gel. One representative experiment of two performed is shown.
Identification of mi protein in mast cells. MC-9 cells were labeled with [35S]-methionine. mi was identified by preincubating the antibody with or without the mi peptide before the addition of the antibody to the cell lysates (A). Immunoprecipitates of [35S]-methionine–labeled mi from either MC-9, spleen, or liver cells are shown in (B). Immunoprecipitates of [35S]-methionine–labeled mi from either BMMC or BM cells are shown in (C). Immunoprecipitates were analyzed by SDS-PAGE. One representative experiment of two performed is shown.
Identification of mi protein in mast cells. MC-9 cells were labeled with [35S]-methionine. mi was identified by preincubating the antibody with or without the mi peptide before the addition of the antibody to the cell lysates (A). Immunoprecipitates of [35S]-methionine–labeled mi from either MC-9, spleen, or liver cells are shown in (B). Immunoprecipitates of [35S]-methionine–labeled mi from either BMMC or BM cells are shown in (C). Immunoprecipitates were analyzed by SDS-PAGE. One representative experiment of two performed is shown.
Characterization of mi's transcription start site in mast cells.Experiments with modified RACE technique showed that the 5′ part is different in the heart as opposed to melanocyte mi mRNA.13 Because a different start site implies the use of a different promoter, the location of the mast cell mi mRNA transcription start site was explored by using the primer extension method. The 5′ labeled primer synthesized from the common part of the heart and melanocyte mi mRNA13 was used in these experiments. As shown in Fig 3, the mast cell mi mRNA begins at the same transcriptional start site as that of melanocytes and around 190 bp from the 5′ end of the primer. The heart mRNA started from the same 5′ end, in contrast to what was previously reported.13
Kinetics of mi protein expression in activated mast cells. Immunoprecipitates of [35S]-methionine–labeled mi derived from IL-3–stimulated (A), IgE-DNP–stimulated (B), or IL-4–stimulated (C) MC-9 cells, using antimouse mi antiserum. Duplicate samples are presented. One representative experiment of three performed is shown. Immunoprecipitates of [35S]-methionine–labeled myosin derived from IL-3–stimulated MC-9 cells using antimyosin antiserum is shown in (D). One representative experiment of two performed is shown. Samples were analyzed by SDS-PAGE.
Kinetics of mi protein expression in activated mast cells. Immunoprecipitates of [35S]-methionine–labeled mi derived from IL-3–stimulated (A), IgE-DNP–stimulated (B), or IL-4–stimulated (C) MC-9 cells, using antimouse mi antiserum. Duplicate samples are presented. One representative experiment of three performed is shown. Immunoprecipitates of [35S]-methionine–labeled myosin derived from IL-3–stimulated MC-9 cells using antimyosin antiserum is shown in (D). One representative experiment of two performed is shown. Samples were analyzed by SDS-PAGE.
Characterization of the rabbit antimouse mi antibody.A peptide specific for a sequence from the c-terminus of the mi protein was chosen on the basis of its uniqueness compared with other known bHLH-ZIP protein sequences. We were able to confirm the identification of mi both with or without preincubation of anti-mi with the nonradioactive mi peptide before the addition of the antibody to the lysate (Fig 4A). It was found that the molecular weight of mi in MC-9 cells is approximately 48 kD, which is similar to that of the murine USF2.20 Because it was reported that mi mRNA is hardly expressed in the liver,3 the expression of mi protein in mouse liver and spleen cells was then determined. As shown in Fig 4B, mi protein was expressed in spleen cells but not in liver cells. As expected, the mast cell mi protein was similar in size to that expressed in melanocytes (data not shown).
Expression of mi protein in mast cell differentiation.The expression of the mi protein in primary culture of mouse BMMC was compared with its expression in BM cells themselves. As can be seen from Fig 4C, mi was clearly expressed in BMMC, whereas it was hardly detected in the BM cells.
The induction of mi protein synthesis by IL-3, IL-4, and IgE-Ag.Because none of these stimuli induced an increase in the level of mi's mRNA, changes in its protein synthesis were further explored in mast cells stimulated by one of the above-mentioned stimuli. As shown in Fig 5, all of these stimuli induced mi protein synthesis. Exposure of mast cells to IL-3 resulted in an increase of mi protein synthesis that started 15 minutes poststimulation (Fig 5A; 5-fold above control ± 0.2 [SE], n = 3, as determined by light-scanning densitometer). Mast cell activation by either aggregation of FcεRI (Fig 5B) or with IL-4 (Fig 5C) also resulted in mi protein synthesis but with slower kinetics, with mi protein synthesis beginning 30 minutes after stimulation with IgE-Ag (7-fold above control + 1 [SE], n = 3, as determined by light-scanning densitometer) or IL-4 (6-fold above control ± 2 [SE], n = 3, as determined by light-scanning densitometer) stimulation. No increase in the synthesis of myosin synthesis was observed, regardless of the stimuli used (data shown only for the IL-3 trigger; Fig 5D).
Stability of the mi protein.Pulse chase experiments showed that mi is a stable protein over a period of 120 minutes after [35S]-labeling (Fig 6). The increase in the level of the [35S]-mi observed in cells that were exposed to the various stimulators is therefore due to the induction of mi protein synthesis rather than to an increase in its stability.
Pulse chase experiment. MC-9 cells were incubated for 30 minutes at 37°C in DMEM methionine-free medium containing 50 μCi/mL [35S]-methionine, washed with RPMI 1640 medium, and incubated for various periods of time in RPMI 1640 medium. Zero minute represents the time when the incubation was started after the pulse chase was performed. The [35S]-mi was immunoprecipitated as described in Fig 4. One representative experiment of three performed is shown.
Pulse chase experiment. MC-9 cells were incubated for 30 minutes at 37°C in DMEM methionine-free medium containing 50 μCi/mL [35S]-methionine, washed with RPMI 1640 medium, and incubated for various periods of time in RPMI 1640 medium. Zero minute represents the time when the incubation was started after the pulse chase was performed. The [35S]-mi was immunoprecipitated as described in Fig 4. One representative experiment of three performed is shown.
Association between mi and USF2.We have recently described the ability of USF2 to associate with c-Fos in IgE-Ag–activated mast cells.20,21 Because the mi gene encodes a bHLH-ZIP protein transcription factor that recognizes an almost identical DNA sequence to that recognized by the USF2,14,20,21 the possible interactions between these two transcription factors on mast cell activation was determined. The lysates from [35S]-methionine–labeled cells were immunoprecipitated on protein A beads with antibody against mi (Fig 7A and B) or with our recently produced USF2 antibody.20 No cross-reactivity was observed between mi and USF2 antibodies. The immunocomplexes were then dissociated and diluted, and the samples were reprecipitated on protein A bound to the antibody that was not used for the first immunoprecipitation (either USF2 antibody [Fig 7A] or mi [Fig 7B]). Reimmunoprecipitation with myosin antibody (Fig 7C) was used as control for the adequacy of the reimmunoprecipitation methodology. A complex containing mi bound to USF2 was clearly detected in the mast cells activated by each of the stimuli in both kinds of reciprocal experiments. This complex was not detected in resting mast cells (data not shown). A problem that occasionally occurred in running samples on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was that the precipitates from the second immunoprecipitation gave crescent-shaped bands, which ran faster than the straight band obtained with the first immunoprecipitate. This gave the impression that the size of mi and USF2 obtained with the second antibody are smaller than that of the same proteins obtained with the first antibody.
Association of mi with USF2 in activated MC-9 cells. [35S]-methionine–labeled activated cells (IL-3, IgE-DNP, or IL-4) were lysed with RIPA and incubated with protein A-bound anti-mi antibody. Immunocomplexes were then dissociated from the beads and either reprecipitated or not on protein A bound USF2 (A). Alternatively, [35S]-methionine–labeled IL-3–activated MC-9 cells were lysed with RIPA and incubated with protein A-bound anti-USF2 antibody. The immunocomplexes were then dissociated from the beads, diluted, and reprecipitated on either protein A-bound mi (B) or myosin (C) antibodies. Analysis was by SDS-PAGE. One representative experiment of three performed is shown.
Association of mi with USF2 in activated MC-9 cells. [35S]-methionine–labeled activated cells (IL-3, IgE-DNP, or IL-4) were lysed with RIPA and incubated with protein A-bound anti-mi antibody. Immunocomplexes were then dissociated from the beads and either reprecipitated or not on protein A bound USF2 (A). Alternatively, [35S]-methionine–labeled IL-3–activated MC-9 cells were lysed with RIPA and incubated with protein A-bound anti-USF2 antibody. The immunocomplexes were then dissociated from the beads, diluted, and reprecipitated on either protein A-bound mi (B) or myosin (C) antibodies. Analysis was by SDS-PAGE. One representative experiment of three performed is shown.
DISCUSSION
The pivotal role played by mi in mast cell development was shown by the use of mice containing the mutated mi gene. However, the regulation of mi expression in these cells has not been investigated until now. In the present study, we show that the expression of mi in mast cells is stimuli-dependent and regulated at the translational level.
The first step in our study was the analysis of the mi mRNA type expressed in mast cells. It was recently reported that two different 5′ cDNA fragments corresponding to different 5′ parts of the mi mRNA were cloned from murine heart and melanocytes using the RACE technique.13 This technique includes several steps after the reverse transcription of the mRNA, including PCR amplification. Thus, it is not the ideal method for locating the mRNA transcriptional start site. To do this for the mast cell mi mRNA, we chose the primer extension method, which is a more appropriate approach for that purpose, because the products of the reverse transcriptase reaction are directly analyzed without any further manipulations. The primers chosen for these experiments were from the common region of the melanocyte and heart mi cDNA.3 13 We found that the mi mRNA in mast cells has a similar 5′ region as that of the melanocyte mi (Fig 3). However, the possibility still exists that there is another transcription start site for the mi mRNA in mast cells that is located further upstream to the melanocyte mRNA transcription start site and therefore could not be detected in our experiments.
The direct involvement of mi in the transcriptional regulation of mast cell specific genes has only begun to be explored. For instance, the expression of mast cell protease 6 is markedly reduced in mi/mi mice,23 probably due to the lack of binding of the mutated mi to its hexameric motif.24 In this way, the stimuli dependent induction of mi synthesis may affect the expression of several other genes that are important for mast cell functions. Furthermore, indirect evidence in support of the idea of mi as a key player in c-kit–mediated signaling has been reported by Dubreil et al.25 They showed that the granulocyte colony-stimulating factor (G-CSF ) receptor could substitute for c-kit in c-kit–deficient mast cells, thus allowing c-kit–deficient mast cells to proliferate in response to G-CSF. However, the addition of G-CSF to G-CSF receptor-transfected mast cells developed in culture from mi/mi mice did not induce their proliferation. Therefore, mi probably acts downstream to c-kit in the growth factor-mediated signaling of mast cell proliferation. A direct role of mi in the regulation of the expression of c-kit was recently shown.26 Thus, it would seem that mi might play a complicated role in mast cell activation both as a regulator of c-Kit gene expression and as an essential component in the c-kit–mediated signaling.
It was expected that mi mRNA would increase due to the exposure of mast cells to external stimuli as do many other transcription factors involved in signal transduction, such as c-Fos or c-Jun.20 27 Surprisingly, we found that short-term stimulation of mast cells by IL-3, IL-4, and IgE-Ag was not followed by induction of mi mRNA. We therefore decided to check the next possible level of mi synthesis regulation, ie, that of protein synthesis. We studied the regulation at this level with our newly produced rabbit antimouse mi antibody. This antibody was raised against a peptide derived from the c-terminus part of the mi protein and a search of the SwissProt Data-Bank for mi peptide homology did not show a protein with greater than 20% homology to this peptide. Using this preparation of antibody, we showed that all the stimuli, although failing to induce mi's mRNA, significantly increased the level of the newly synthesized mi protein within a relatively short period of time without affecting the level of myosin. Pulse chase experiments showed that the half life of the mi protein in uninduced mast cells is significantly longer than the time needed for a substantial increase in the amounts of newly synthesized mi protein. Accumulation of mi protein was therefore most probably a result of its increased rate of synthesis. Such translational regulation of mi may allow rapid increases in its availablity for its specific functions and therefore can affect the general cellular transcriptional control network.
The role of mi as a transciptional regulator has been studied mainly in melanocytes. Transient cotransfection of plasmids containg a reporter gene under the regulation of the tyrosinase gene promoter-enhancer and mi or USF-overexpressing plasmids showed that mi and USF are able to transactivate this promoter in melanocytes.28 Furthermore, although the reduced expression of mi in E1a transformed melanocytes correlated with decreased tyosinase expression in these cells, the overexpression of this transcription factor led to the reexpression of this important enzyme in melanin production.29 Therefore, transient increases in the expression of this gene can cause important cell specific alteration in gene expression in melanocytes.
mi participates in the coordination of gene expression in mast cells either directly, by binding to DNA binding sites, or indirectly, through its effects on the activities of other transcription factors. We have shown previously that IgE-Ag induces the synthesis of USF2, another bHLH-ZIP transcription factor in mast cells.20 As an initial step towards a better understanding of the role played by mi in mast cell function, we showed that activating mast cells with IL-3, IL-4, or IgE-Ag caused the formation of a mi-USF2 complex (Fig 7). Thus, mi may indirectly affect the activity of other transcription factors in activated mast cells.
We have also followed the expression of mi during mast cell differentiation from BM cells. The relative paucity of mast cells in mi/mi mutant mice and the increase in its expression during mast cell differentiation (Fig 4) provide the strongest evidence of the important role of this gene in mast cell development. Surprisingly, nondifferentiated mast cells can be grown in vitro from the BM of mi/mi mice6,7; therefore, mi is not an essential gene for the initial cellular commitment to the mast cell lineage. Therefore, mi might play an important role in the differentiation, proliferation, and survival of committed mast cells as a key molecule in response of these cells to various external stimuli. Recently, several studies have provided us with information regarding the mast cell progenitor population.30 31 Study of mi's expression in this cell population could provide important insights into the role of mi in early mast cell differentiation events.
In summary, the results presented here show that mi is translationally induced by several external stimuli in mast cells and can bind to USF2, another stimuli-induced transcription factor. Therefore, it is likely that translationally induced mi has an important role in the mast cell transcriptional response to various stimuli.
H.N. and Z.Z. contributed equally to this work.
Address reprint requests to Ehud Razin, PhD, Department of Biochemistry, Hebrew University-Hadassah Medical School, PO Box 12272, Jerusalem, 91120, Israel.

![Fig. 2. Kinetics of c-fos mRNA accumulation in activated mast cells (IL-3 [A] and IgE-DNP [B]). Northern blot analysis was performed as described in Fig 1. One representative experiment of two performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f2a.jpeg?Expires=1769367621&Signature=hzsrngLDHsupP0WR1EE83pjP6gGpdiRsn-Oc17w5mHSgansbXNlVr5IZ9fWE7h~gAi0Pfq-LUi-tX6ILP2x7o6PpcdbzV9ZSV4Le73YluYWUadUE23FOmjrsCX6XZE86oj6ZejfWxfremRo9AvbX309AA-y-DjtSGsCoEGH1jeFcBqSY9eaxUgkcYpldvcV06T-ikGWIp~3nt96~BwUQeo6fYpTyVUV-wna4Br8phtTEHf66D~uE4wPRCsf5jEL2DaYS91YYHenVPZ0GAD4P2LctaMCeJECHlPfkPYX1XpWgMPgA-YwyElYsGneAfivQUTdfZiuO633EgRipjQDP9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Kinetics of c-fos mRNA accumulation in activated mast cells (IL-3 [A] and IgE-DNP [B]). Northern blot analysis was performed as described in Fig 1. One representative experiment of two performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f2b.jpeg?Expires=1769367621&Signature=nS6N81zYDc00VNqbMHfcYAK5rCWDBaGEeqqRAA1kAmJxVJb6-xdAM~Sgq0tsWlkxZIEmm5z3LnXKiwjiLq8CKZR3KqVxVgwRfue0lKB~DizBDmxfbcDdDeUMyj12uH7Z5srVxHN4lH3kcM4V3Pi8lFrguGt31OfInR0-Em5QzX8IsAVqSFyhjxZR7p3zN~Z4O2YQfbB1RhL0A3xGLu6V~5dkmQMfkUf1oTZ8m~wApJpymM2aTnsS47n5VfTquvifsrw39WZdjTumo61XZ1rI1t~gd4EQbIakoVm8-zInuh9JmyrdOaXVvP2qf--35ocUoL3x0A5Q0bZ1mFHB1MUVbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. mi mRNA starting site (primer extension experiments) in mast cells. An oligonucleotide 5′ GTT TTC CAG GTG GGT CTG CAG 3′ from the putative common part of the heart and melanocyte mi mRNA was end-labeled and then the mRNA was precipitated and reverse transcribed as described in Materials and Methods. The samples were then loaded together with a puc18/Msp I-restricted [32P]-labeled size marker on a 4% acrylamide 7 mol/L urea denaturing gel. One representative experiment of two performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f3.jpeg?Expires=1769367621&Signature=APme0CCrx4OF8Omb37MEbvZ4DJVMh1xPCBB96Pim2ZRGCbhbkv68BtStOjS34BOd3EuE3zh8wsdrmfHsHAUK8z-IhsRTFIhNtuiUDhyt0Eb7~x6rU0z83hV66IOmqAKw2yegb0EkZQscOwjKaO4XiHNQdWUhfzSlGebhgalUz8UEPslF9PyGWFyknvzTwcwZB0-8NAN1r2YU02Sz~tspBjJlIn-3ZH5X17ayxvRZkZKd114P3Z9yOMOEkb5-vX2oVxmbODof5VkK07ekTg0fPYC0CbaJNiXkGZCE5LvBhPlA0geD1XU7D9kp-1MPGjVMwdMWYZWcziGvG8g8m3wF5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Identification of mi protein in mast cells. MC-9 cells were labeled with [35S]-methionine. mi was identified by preincubating the antibody with or without the mi peptide before the addition of the antibody to the cell lysates (A). Immunoprecipitates of [35S]-methionine–labeled mi from either MC-9, spleen, or liver cells are shown in (B). Immunoprecipitates of [35S]-methionine–labeled mi from either BMMC or BM cells are shown in (C). Immunoprecipitates were analyzed by SDS-PAGE. One representative experiment of two performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f4a.jpeg?Expires=1769367621&Signature=rfBb31DhPiIhy-95B-Il4qTiZyWLrkr3LioRNusvBfVEKq9uPAOw0jw-ka5VS6EzzzXasBv1WhDWAhU779ORlUxL8KJHL6nkjR1IGUpvKTIT~oCq7Gipf8Cl02IXxpzZo2X5I4QBmzYC3z1KVSPiHVupzAJ-1XoGOWFEPabqroDENgmShgvU0YKCUgzrpqlyF4E5TCnO-YK6naj39GvYVEszTsGYwWnSxbfIWY87m7~IdeW4wbr~Ndq2NLzSUFqYtpwTe27-TttP-U4hzPjpwvoV5VYLJ7Lx5QmNmc5HYrB9Vm3rrGTrXOppbguQBdE9cONPZkUIgtbUw1rZgylQvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Identification of mi protein in mast cells. MC-9 cells were labeled with [35S]-methionine. mi was identified by preincubating the antibody with or without the mi peptide before the addition of the antibody to the cell lysates (A). Immunoprecipitates of [35S]-methionine–labeled mi from either MC-9, spleen, or liver cells are shown in (B). Immunoprecipitates of [35S]-methionine–labeled mi from either BMMC or BM cells are shown in (C). Immunoprecipitates were analyzed by SDS-PAGE. One representative experiment of two performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f4b.jpeg?Expires=1769367621&Signature=uYMyvHTGCrR378Luq1jcdzaQ6OMkASF7nmu6WAaDt6Ba-ke9AdOaGD0NHc~At11WiOzDufrwhs7-Ad6G0QtxEz~i1trKXYkegQorY7X6ZSzZgdlCfwilT3ZXZWahxCKlJVhm-oDY~Serlb8Wfk5isIdFhq3BHF2bJuex14ow3saDTkzXlmweixZSQF9EiW0aZ-ciBgQmUwzPXV54Q3plfnMMoakpO2shyQ~oC8udmENCxfslpsm-yiD~dfH5gboVSQfBv2AC26Nhd2pLPDBrfFnZk4S0~nCRZpauvDYjd8gncBiXCOwEyK5FU9SIGhwA6ZL35O~qW0m8P5gyZdM6CA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Identification of mi protein in mast cells. MC-9 cells were labeled with [35S]-methionine. mi was identified by preincubating the antibody with or without the mi peptide before the addition of the antibody to the cell lysates (A). Immunoprecipitates of [35S]-methionine–labeled mi from either MC-9, spleen, or liver cells are shown in (B). Immunoprecipitates of [35S]-methionine–labeled mi from either BMMC or BM cells are shown in (C). Immunoprecipitates were analyzed by SDS-PAGE. One representative experiment of two performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f4c.jpeg?Expires=1769367621&Signature=OPviKFQvDErixuOYC0lDj0eE~mOMCS35zxfdUIDooV5fUeWxCvyXGQfA-N7TaCfMEDZM2S8a9Chao8~uD8HpaI8tndpnTAugjh8pd8Gl-DnDYkrYW5LZO4C7JbOGxlb1DKRLo5BCwVy9RteusrcFa~jfxiHufb-G6JU8is~y9cwdTGAX3KnfTiv7xDhG1StYP46UZleZYRs49G71iGtEOq-wMBGYB4376Xw6Qm4Y6zSDbOyRXD3mk8DG3yjFlHECDVLEq2zq0-Bcy3U13PQZQRwETeMRLKg9XMQoAfQERidIXKOsJzA0ek8aBzXiZYcOe-ZkfWq1~4n5EQze56CfZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Kinetics of mi protein expression in activated mast cells. Immunoprecipitates of [35S]-methionine–labeled mi derived from IL-3–stimulated (A), IgE-DNP–stimulated (B), or IL-4–stimulated (C) MC-9 cells, using antimouse mi antiserum. Duplicate samples are presented. One representative experiment of three performed is shown. Immunoprecipitates of [35S]-methionine–labeled myosin derived from IL-3–stimulated MC-9 cells using antimyosin antiserum is shown in (D). One representative experiment of two performed is shown. Samples were analyzed by SDS-PAGE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f5a.jpeg?Expires=1769367621&Signature=RS7Pdxh0xW9jpUttp9vBNmGxHtMNCMBjjf7BDx6nSLJbO-ETq7tiyOjwg8jFM4JwBF7SuIAcfKvmzF6Lin5-37RM7QKt3P8c4pCGWLYkIZs371dYOY7bhuo-IcPseyn9e1WnoYEMv~zybSKhseLtepa8OBnSnxS6oQbZ9JCB~~DEuxXfUTiS-M~wkrfUwOt2vTdc~BsHV5l5Y7725ZyAXcP1J~lO2mQ6gEevGjHRzygO7prRqj1x5w7qLS9B0GH1tP2aKM9B0ObV-VbKrUeiZ4xevgOnDOR6cYWJXh6vU-PzuSh1kCqKZ2XV4PTFANd1~WeolMVLvcInOg6pfeENXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Kinetics of mi protein expression in activated mast cells. Immunoprecipitates of [35S]-methionine–labeled mi derived from IL-3–stimulated (A), IgE-DNP–stimulated (B), or IL-4–stimulated (C) MC-9 cells, using antimouse mi antiserum. Duplicate samples are presented. One representative experiment of three performed is shown. Immunoprecipitates of [35S]-methionine–labeled myosin derived from IL-3–stimulated MC-9 cells using antimyosin antiserum is shown in (D). One representative experiment of two performed is shown. Samples were analyzed by SDS-PAGE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f5c.jpeg?Expires=1769367621&Signature=YuWMjn2IoPjgEcWdiq9qVyXqNmre~mxvpykcwYNe4rMLEwQH0AzL~OlRlPWpQVGV-SQPxUk6r-z7dyVBdu6ugm8dQxEf6li4o3TzL8FXM4~lISZlPssaPPFMkVsdbSkiT9kJtSmrV0jQlLET-yWfbTozshT6icJ0oy-pRdBu9q3mKhGZ-nmzbm7aYqT-d~nXcVhB0UGplTE3b3D5JUA9oyldRCIuHZtaazBAGe1kKP7456Z05IGsZwKSv7w1cz5K293qRtBaf4VKkGyxW7yz-9MZ5wEZRcvhg5rgyjoAE5JyPSA6X9HGJ~Zd6dnKiI~CUkgVq7CsBOsl02zCpFPfBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Kinetics of mi protein expression in activated mast cells. Immunoprecipitates of [35S]-methionine–labeled mi derived from IL-3–stimulated (A), IgE-DNP–stimulated (B), or IL-4–stimulated (C) MC-9 cells, using antimouse mi antiserum. Duplicate samples are presented. One representative experiment of three performed is shown. Immunoprecipitates of [35S]-methionine–labeled myosin derived from IL-3–stimulated MC-9 cells using antimyosin antiserum is shown in (D). One representative experiment of two performed is shown. Samples were analyzed by SDS-PAGE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f5d.jpeg?Expires=1769367621&Signature=nHs2PiCxAbIwz1pbAYVJboUWq5iByYDzMkbZp5TIliG-rrj~UcNU0whrrkyt7Gsb70Eg07brrnRyd~hqS7NLVt9TxNrRJkGF0XWcCVhF0r3t9XT7m8B2qgP7z3XkGlD8XyYzp8Y8JlP3W02PHGtd7qAqldoe-NILjBK4-x35g-wK3tabW7EMpe~8RLHZgWT1kY7-vX8iqBXFzO4t4fBluR~64BQlZzvQqFRx-~2w9pTEbFFnhaOgBJ89m3He9-51mkNbVSTXYcHejH~LoKik2Wlboprykf8a-iaQisQGq9e-fnGZJewcp7uvkAT3y54WuWmCIpm0BTxjU~GEVvCSLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Kinetics of mi protein expression in activated mast cells. Immunoprecipitates of [35S]-methionine–labeled mi derived from IL-3–stimulated (A), IgE-DNP–stimulated (B), or IL-4–stimulated (C) MC-9 cells, using antimouse mi antiserum. Duplicate samples are presented. One representative experiment of three performed is shown. Immunoprecipitates of [35S]-methionine–labeled myosin derived from IL-3–stimulated MC-9 cells using antimyosin antiserum is shown in (D). One representative experiment of two performed is shown. Samples were analyzed by SDS-PAGE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f5b.jpeg?Expires=1769367621&Signature=cPodIpHhgOu1H34uUGxjwEwRxPtaz5-JNbPpqmfDmhbavVtl4EGzqMuvCweh4aGIRXreBHBQ3qAZW04iCDp7DM~i6gHvne0UpkjqgQbXouB1K3zcGQs8ht8YoWVK~s7Tzmy~CdBd-rXii~Fk-phIkcu6E15LPPJ0oRymAbJ~cc-2l98CmEHI6A7FjVIwk~Pd7X6hs-GtocgCIBmLWLxZg4PiPoAKHZo09yWq6pCWNiWYQst4aQj-lvJ7wNRpJ8wPQw9MGiy4I5~FefWDwIUTSirGCmOnhAYg-uk9lLCmhnGcrVW54OMTzvEyK54Vko0hae0kK1WxT4zMylRd3zQ~HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Pulse chase experiment. MC-9 cells were incubated for 30 minutes at 37°C in DMEM methionine-free medium containing 50 μCi/mL [35S]-methionine, washed with RPMI 1640 medium, and incubated for various periods of time in RPMI 1640 medium. Zero minute represents the time when the incubation was started after the pulse chase was performed. The [35S]-mi was immunoprecipitated as described in Fig 4. One representative experiment of three performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f6.jpeg?Expires=1769367621&Signature=Ec1iIv7ISXAjK9nrdDkO8fPcPFAcIYPw57dkRZ-ccF28pGoTMPhWkM00ws-Q8pKPbOye~9Btpz~OrdNk0YLYSkZrRNFcAd1xbY8Np9Qk1xaj9Hoo9TmFMlQ8mcQTKH6vJWtEWANM9GOhkpVrhMlUbhyhgycuf1RsBNTCyUyzDZtko1RPieDyI06D7sAgBZv~JO78C1HuuwOlfwH50SJ4Qq~DK~mRi~4Elat32xLADPd5U95qscP55IMKMYcTvChWlXEumQoFewZfereVxVkpoQG5lUfBkF6iifZZ1NunWkNnOIpc-uB0L0z0Q6WspvmFviu-~7ozie8CK9zA4YuFpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Association of mi with USF2 in activated MC-9 cells. [35S]-methionine–labeled activated cells (IL-3, IgE-DNP, or IL-4) were lysed with RIPA and incubated with protein A-bound anti-mi antibody. Immunocomplexes were then dissociated from the beads and either reprecipitated or not on protein A bound USF2 (A). Alternatively, [35S]-methionine–labeled IL-3–activated MC-9 cells were lysed with RIPA and incubated with protein A-bound anti-USF2 antibody. The immunocomplexes were then dissociated from the beads, diluted, and reprecipitated on either protein A-bound mi (B) or myosin (C) antibodies. Analysis was by SDS-PAGE. One representative experiment of three performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f7a.jpeg?Expires=1769367621&Signature=LcsCAB9Z64FvcvvrEPTFtrFlau8HpoHc6VAM1H0R0Fe-9HthS9xSzsjtp9zrleU5KApkxN~4Sn28EyA3PPeEbM~2wSLJC-RsvooOEgOaxbTUbMV7uw4MUEHk1VUWr9YENKy5BLnC0k95GnNZkap5iIL1acDcjDMv3Mzhr46j8VxOCc-ZgJrSmnznxybeekqgzJg2ELUGg62BV15OzA6vU4fLo-REIIvwhGAONqn7pHwCvzMt~blg-qizTt2ZsiFJKPhKgox--swSySD7y-j9qR8A3YAR5N8m3-UHoGcxOvd0ydWHDBXEOcfeYITsJFUrPy7fthyO3-GVzFTD-TIJCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Association of mi with USF2 in activated MC-9 cells. [35S]-methionine–labeled activated cells (IL-3, IgE-DNP, or IL-4) were lysed with RIPA and incubated with protein A-bound anti-mi antibody. Immunocomplexes were then dissociated from the beads and either reprecipitated or not on protein A bound USF2 (A). Alternatively, [35S]-methionine–labeled IL-3–activated MC-9 cells were lysed with RIPA and incubated with protein A-bound anti-USF2 antibody. The immunocomplexes were then dissociated from the beads, diluted, and reprecipitated on either protein A-bound mi (B) or myosin (C) antibodies. Analysis was by SDS-PAGE. One representative experiment of three performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f7b.jpeg?Expires=1769367621&Signature=ZiGRBWWuBk20cQPualIXTTFZ1oAAVAEI8Pq6O9GvB8vy-lQSoBb4C9lrxd8k814CNiH8OUpSxvh3R-x5~u498oGh7TaDqCyM8MjEgiXFv1L2ky-vd3h4sYEOMZPMyGsnqUS1HgP5tFr7zzuy27j0waj8IRE44IRZS1ayR0RInThQrhxtF7UcP0aWrph4NEwNNke6CwsQKtBSE2GaRAZkB66~lsLBvrtX~c7MrGLsFNJ4~S-LLqMfSAJt-x5Lm6f2Efhr-Jl6YvHmMkkOSA5E6o79WxhPa4IrmoKYBPU2XXOTsRZI3aX9-yH23pxHhBYbMxUFxGsTpCrJ-qaTOcG8NQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Association of mi with USF2 in activated MC-9 cells. [35S]-methionine–labeled activated cells (IL-3, IgE-DNP, or IL-4) were lysed with RIPA and incubated with protein A-bound anti-mi antibody. Immunocomplexes were then dissociated from the beads and either reprecipitated or not on protein A bound USF2 (A). Alternatively, [35S]-methionine–labeled IL-3–activated MC-9 cells were lysed with RIPA and incubated with protein A-bound anti-USF2 antibody. The immunocomplexes were then dissociated from the beads, diluted, and reprecipitated on either protein A-bound mi (B) or myosin (C) antibodies. Analysis was by SDS-PAGE. One representative experiment of three performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/8/10.1182_blood.v89.8.2999/4/m_bl_0007f7c.jpeg?Expires=1769367621&Signature=SdzLW3OlFA7gYzoAVfCbvFtbAbD1f8YwpmfXq398z5DU6IoZ5aiYyVIRvKDYCeskq6iLR5tGb3Jo7CScuiFiSNW8VCgvz4qtrtk~NRNoetSPUO~a7VsOp1o0Ffx39d~ByAjq9DzazfOIxYMiU2kM6aUcAnjzFzSm-vACV1-I1GB4Xod5ZTOBS4Zmx3Gj1SgZqSxYS0Ultd7r971dWHSJiRwmaAS92vhw5U~Gfn10qhZYILudgNrbEbCwpIVt--yt1pAPxFRRE4obyz4G0qyANsDXDeDdI6DyV69xUu4TaIwmyfDycEgGpNiVblitr~CBoKSkkau~MdqXJAu-4liD9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal