To the Editor:
An association between hemolytic favism and ACP1 genotype in male subjects from the populations of Rome and Sardinia was reported by our group in 1971,1 2 but it was not possible at the time to propose a fully satisfactory explanatory mechanism. Since then, a number of important findings regarding the enzymatic properties of ACP1 and the biochemistry of hemolysis have been obtained, suggesting a biochemical mechanism for such association.
Band-3-protein (B3P) may regulate the glycolytic rate and its clustering plays a central role in the chain of events leading to erythrocyte destruction.Phosphofructokinase, aldolase, glyceraldehyde-3-phosphate-dehydrogenase, and catalase have binding sites near the N terminus of the cytoplasmic domain of B3P. A tyrosine residue at position 8 is a substrate for various tyrosine-kinases and its phosphorylation promotes displacement of glycolytic enzymes representing a possible regulatory mechanism for glycolytic activity.3 4
Hemichromes have a high affinity for the cytoplasmic fragment of B3P causing a clustering of band 3 and other proteins in red blood cell (RBC) membrane; once a transmembrane molecule is clustered at one side of the membrane, the binding of multivalent ligands on the other side of the membrane is enhanced by several orders of magnitude. Clustering of band 3 enhances the binding of autologous IgG with anti B3P specificity: this is followed by activation of the alternative complement pathway and generation of C3b fragments which binds to damaged RBC. Intracellular signals for destruction of damaged RBC may develop in a variety of ways even without extensive Heinz-bodies formation.3
ACP1 is a phosphotyrosine-phosphatase (PTPase) having two isoforms, f and s, with different biochemical properties and, probably, different functions.Each of the human homozygous types ACP1 *A/*A, *B/*B, and *C/*C, shows two main isozymes, designated f and s: the first two columns in Table 1 show the concentrations of f and s isozymes for each ACP1 genotype.5 Experimental evidence suggests that the two isozymes elicit some structural differences in the catalytic sites and have different cellular target substrates.6
Boivin and Galand7 have shown that ACP1 dephosphorylates a tyrosine residue of erythrocyte membrane protein 3 (B3P). Phosphorylation of tyrosine of B3P prevents binding of several glycolytic enzymes and results in elevated glycolytic rates in erythrocytes.8
ACP1 f isoform is less stable than s isoform under the action of oxidative drugs.The different stability of the two ACP1 isoforms under the action of oxidized glutathione (GSSG) and acetylphenylhydrazine (APH) was described 2 decades ago in a number of reports by our group9-11: in all conditions, s isoforms of the various phenotypes are found to be more stable than f isoforms.
ACP1 f isoform is more efficient than s isoform in dephosphorylating B3P.Stefani et al6 have recently shown that the synthetic phosphotyrosine-containing peptide corresponding to the 5-16 sequence of B3P is more efficiently hydrolyzed by the f isoform than by the s isoform. Thus, a decreased concentration of f isoform of ACP1 may enhance tyrosine phosphorylation at the cytoplasmic tail of band 3. This would decrease the binding of glycolytic enzymes aldolase, phosphofructokinase, and glyceraldeide-3-phosphate dehydrogenase, resulting in an enhanced rate of glycolysis. It could also induce an increased tendency to conformational changes of B3P which may favor clustering and in turn the binding of multivalent ligands to the external side of B3P. This last possibility lacks experimental support at present, but seems worthy of exploration.
Susceptibility to favism is higher in G-6-PD–deficient subjects with low concentration of ACP1 f isoform.The order of susceptibility to favism is *B/*B < (*A/*B, *B/*C) < (*A/*A, *A/*C).1 This pattern cannot be explained on the basis of the enzymatic activity on paranitrophenylphosphate because this activity is in the order *A/*A < *A/*B < (*B/*B, *A/*C) < *B/*C. When assigning concentrations of ACP1 isoforms to genotypes according to Dissing,5 a significant negative correlation can be noted between the concentration of f isoform and the susceptibility to favism (Table 1). This closely corresponds to the above-mentioned ordering of ACP1 genotypes with respect to their susceptibility to favism. On the other hand, the concentration of s isoform does not appear to be correlated with susceptibility to favism.
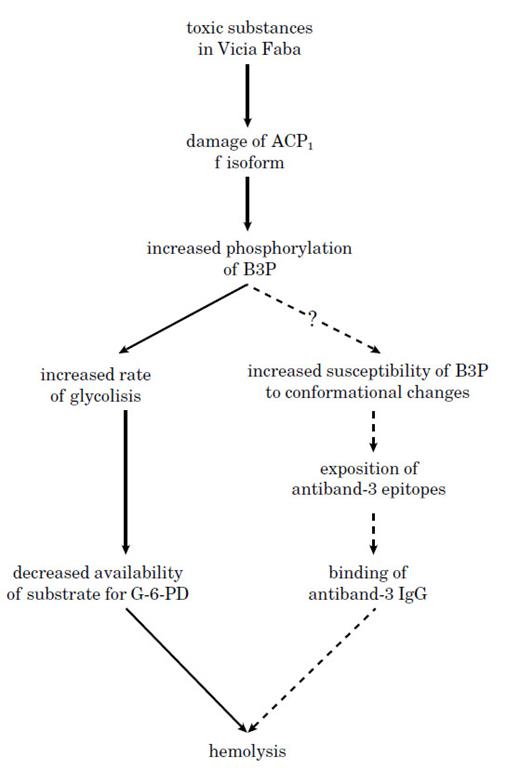
The possible role of ACP1 genetic variability in the sequence of events leading to hemolysis.A plausible mechanism underlying the association of ACP1 with favism is depicted in Fig 1. Inactivation of ACP1 f isoform following the action of toxic substances from Vicia Faba would lead to a relative increase in B3P phosphorylation. This may cause a decrease of the substrate for G-6-PD and/or a possible increased tendency to conformational changes in B3P which could precipitate the RBC destruction. Subjects with genetically determined low concentration of f isoform would be more susceptible to the effects of Vicia Faba as compared to genotypes with high f isoform concentration.
A possible mechanism explaining the association between ACP1 and hemolytic favism.
A possible mechanism explaining the association between ACP1 and hemolytic favism.
Experimental models investigating ACP1 stability under the action of oxidative drugs11 have shown that the f/s ratio is substantially decreased during the early stages of oxidative damage. This may be important at the functional level. For example, ACP1 genotypes *A/*A and *A/*C have very similar concentrations of f isoform but very different f/s ratios (2.4 v 0.6), and it can be noted that in both Rome and Sardinia, the susceptibility to favism is higher for *A/*C than for *A/*A. Moreover, we have previously shown12 that neonatal jaundice, in which oxidative hemolysis may play an important role, is more common among newborns with the *A/*C genotype than among those with the *A/*A genotype.