Abstract
Whereas unperturbed endothelial cells provide potent anticoagulant properties, exposure to inflammatory and atherogenic stimuli can rapidly lead to a procoagulant behavior. Because recent studies provide evidence that apoptosis of vascular cells may occur under conditions such as atherosclerosis and inflammation, we investigated whether apoptotic endothelial cells may contribute to the development of a prothrombotic state. In this report, it is shown that both adherent and detached apoptotic human umbilical vein endothelial cells (HUVECs) become procoagulant. Apoptosis was induced by staurosporine, a nonspecific protein kinase inhibitor, or by culture in suspension with serum deprivation. Both methods resulted in similar findings. As assessed by flow cytometric determination of annexin V binding, HUVECs undergoing cell death exhibited typically a more rapid exposure of membrane phosphatidylserine (PS) than DNA fragmentation. Depending on the stage of apoptosis, this redistribution of phospholipids was found to induce an increase of the activity of the intrinsic tenase complex by 25% to 60%. Although apoptotic cells did not show antigenic or functional tissue factor (TF ) activity, when preactivated with lipopolysaccharide, TF procoagulant activity increased by 50% to 70%. At 8 hours after apoptosis induction, antigenic thrombomodulin, heparan sulfates, and TF pathway inhibitor decreased by about 83%, 80%, and 59%, respectively. The functional activity of these components was reduced by about 36%, 52%, and 39%, respectively. Moreover, the presence of apoptotic HUVECs led to a significant increase of thrombin formation in recalcified citrated plasma. In conclusion, apoptotic HUVECs, either adherent or in suspension, become procoagulant by increased expression of PS and the loss of anticoagulant membrane components.
VASCULAR ENDOTHELIUM is known to provide potent anticoagulant properties, preventing both the initiation and propagation of the coagulation process. Several endothelial cell-mediated mechanisms have been well characterized and found to be responsible for the maintenance of the antithrombotic state. In particular, endothelial cells have been shown to inhibit coagulation by the expression of thrombomodulin (TM), which promotes activation of protein C1; heparan sulfate (HS), which activates antithrombin III (ATIII) to accelerate thrombin inhibition2; release of tissue factor pathway inhibitor (TFPI), which blocks the factor VIIa-TF-factor Xa complex3; and annexin V, which prevents binding of coagulation factors.4 However, endothelial cells can rapidly shift the hemostatic balance from an antithrombotic to a prothrombotic state. There are a number of studies showing that different perturbing agents, such as proinflammatory cytokines (eg, lipopolysaccharides [LPS], interleukin-1, and tumor necrosis factor-α), atherogenic substances (eg, lipoprotein A and homocysteine), hypoxia, hyperthermia, viral infections, and activated leukocytes or their releasates, alter the anticoagulant function of endothelial cells. This may be caused by increased binding and activity of coagulation factors, loss of anticoagulant surface components, or, more actively, by expression and/or release of prothrombotic substances, such as TF, factor V, platelet-activating factor, von Willebrand factor, and plasminogen activator inhibitor (reviewed in Martin et al,5 Nachman,6 and Rodgers7 ).
Apoptosis or programmed cell death is an active process of cell death, morphologically and biochemically different from necrosis, and occurs under physiologic and pathophysiologic conditions. Besides vasculogenesis, apoptosis of endothelial cells is mainly supposed to contribute to vascular pathology, including atherosclerosis, inflammation, and wound healing. Vascular cell death has been found in several studies of atherosclerotic vessels, showing extensive apoptosis of smooth muscle cells.8-10 Although it has not been established to what extent endothelial cell death occurs in vivo, there is increasing evidence that endothelium may become apoptotic under such conditions as well. Years ago, Hladovec et al11-13 detected circulating endothelial cells in blood from patients with hypertension and acute coronary heart disease and in experimental homocysteinemia, suggesting substantial endothelial cell death. Electron microscopic studies of occluded human saphenous vein grafts showed severely abnormal endothelial cell structure with wide intercellular junctions, which was suggestive of apoptosis.8 Moreover, it has been shown that administration of LPS into rats induced massive endothelial cell death in the aortas.14 Further evidence comes from a number of in vitro studies showing that cultured endothelial cells undergo apoptosis upon exposure to various stimuli. Endothelial cell death was found after treatment with tumor necrosis factor-α,15 LPS combined with heat shock16 or ionizing radiation,17 high glucose concentration,18 and low concentrations of hydrogen peroxide.19 Hence, perturbing conditions that induce procoagulant activities of endothelium seem also to be able to cause endothelial cell death. Whereas the coagulation process is thought to be initiated by exposure of subendothelial matrix or TF expressed on extravascular cells, it is not known whether apoptotic endothelial cells are procoagulant as well.
Thus, the purpose of this study was to determine whether there may be a link between endothelial cell death and prothrombotic state, such as may occur in atherosclerosis and inflammation. Specifically, we determined whether endothelial cells undergoing apoptosis, a process of several hours, would lose their anticoagulant properties and become able to initiate coagulation process. Our results show that, during apoptosis, human umbilical vein endothelial cells (HUVECs) become procoagulant due to rapid redistribution of phosphatidylserine (PS) and loss of anticoagulant surface components.
MATERIALS AND METHODS
Cell Culture
HUVECs were obtained by collagenase treatment of umbilical cord veins as previously described.20 Cells were cultured on gelatin-coated or collagen-coated (see further) dishes and propagated in RPMI 1640 medium supplemented with 20% bovine calf serum, 90 μg/mL heparin (Sigma Chemical Co, St Louis, MO), and 50 μg/mL endothelial cell growth factor prepared from bovine hypothalamus.21 HUVECs derived from passage two or three were then cultivated in 6- or 48-well dishes (Costar Corp, Cambridge, MA) and maintained at 37°C in 5% CO2 .
Apoptosis Induction
Apoptosis of HUVECs was induced either by 200 nmol/L staurosporine (Sigma) in growth medium or by culture in suspension with serum deprivation, as previously described.22 To prevent HUVECs from adherence, tissue culture plates were coated with 2 mL of melted 2% agarose (GIBCO-BRL, Life Technologies Inc, Gaithersburg, MD) in RPMI 1640 medium. The cells were then kept in culture medium without heparin, serum, and growth factor.
Flow Cytometry Assays
HUVECs cultivated in 6-well plates were harvested, washed twice with phosphate-buffered saline (PBS) buffer, pH 7.4, and subsequently resuspended in 180 μL HEPES buffer (10 mmol/L HEPES, 1% bovine serum albumin, 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2 , 2.5 mmol/L CaCl2 , pH 7.4). Each step of the staining procedure was performed on ice for 30 minutes, followed by two washing cycles (centrifugation at 300g for 5 minutes). The following final concentrations were used. (1) For annexin V binding, 100 nmol/L fluorescein isothiocyanate (FITC)-conjugated recombinant annexin V was used. Purification of recombinant annexin V and labeling with FITC (1 mol FITC/mol annexin V) was performed as previously described.23 24 (2) For TM, 10 μg/mL antihuman TM monoclonal antibody (MoAb) 3E2 (Alexis Biochemicals Corp, San Diego, CA) was used, followed by 40 μg/mL FITC-conjugated goat IgG-1 antimouse antibody (Caltag Laboratories, San Francisco, CA). (3) For ATIII binding, 100 μg/mL human ATIII (Alexis) was used, followed by 1:200 diluted IgG-1 antihuman ATIII MoAb (Sigma) and 40 μg/mL FITC-conjugated goat IgG-1 antimouse antibody (Caltag). (4) For TFPI, rabbit IgG antihuman TFPI antibody (American Diagnostica Inc, Greenwich, CT) was used, followed by 60 μg/mL FITC-conjugated IgG antirabbit MoAb (Sigma). For TFPI staining, cells were first permeabilized with 0.02% saponin for 10 minutes. After staining of the surface molecules, the cells were fixed in 80% ethanol for 30 minutes, washed twice, and resuspended in 200 μL PBS buffer, pH 7.4, and 0.1% Triton-X 100 (Sigma) containing 5 μg/mL propidium iodide (PI; Sigma) and 50 μg/mL ribonuclease A (R-6513; Sigma) for DNA staining. Specimens were then analyzed by a FACScan (Becton Dickinson, Mountain View, CA). At least 20,000 events were evaluated.
Oligonucleosomal DNA Banding
Internucleosomal DNA fragmentation was shown by the harvesting of total cellular DNA, as previously described.25 Briefly, adherent and detached cells were harvested separately, washed, and lysed with 50 mmol/L Tris, pH 7.5, 10 mmol/L EDTA, 0.5% Triton-X-100, and 0.5 mg/mL proteinase K for 2 hours at 50°C. Samples were then extracted twice with phenol/chloroform/isoamyl alcohol and precipitated with ethanol. The pellet was resuspended in Tris-EDTA and 10 μg/mL ribonuclease A, and the DNA was separated on a 2% agarose gel.
TF Determination
Antigenic TF.Quantitative determination of TF was performed using an enzyme-linked immunosorbent assay (ELISA) kit (American Diagnostica Inc). Cells were prepared as follows. Adherent or detached HUVECs from a 6-well plate were harvested and lysed in PBS, pH 8.5, containing 1% Triton X-100. Cell membranes and nuclei were pelleted by centrifugation at 14,000g for 60 minutes at 4°C. The protein content of the supernatant was determined by a protein assay kit (Bio Rad Laboratory, Hercules, CA) and adjusted to 2 mg/mL. Samples were then used for ELISA according to the manufacturer's instructions. As a positive control, cells were treated with 100 ng/mL LPS (Escherichia coli 0111.B4; Sigma).
Functional TF.Adherent HUVECs in a 48-well plate or HUVECs derived from culture in suspension were washed and incubated with 200 μL of HEPES buffer containing 10 nmol/L purified human factor VIIa (Alexis) and 300 nmol/L purified human factor X (Alexis) at 37°C. At various time points, 25 μL of the mixture was removed and added to 5 μL of PBS buffer with 60 mmol/L EDTA to stop factor Xa formation. Factor Xa activity was then determined by the hydrolysis of the chromogenic substrate S-2222 (Chromogenix, Moelndal, Sweden) at a final concentration of 0.2 mmol/L. The optical densities were read at 405 nm using a plate-reader spectrophotometer (EL-340; Bio-Tek Instruments, Winooski, VT). The amount of factor Xa was calculated by comparison with a standard curve made with known amounts of purified factor Xa (Sigma). Where indicated, cells were treated with 100 ng/mL LPS for 6 hours. When factor VIIa was omitted from the assay, no activity was found in LPS-treated cells.
Intrinsic Tenase Complex Activity
HUVECs (prepared as in the functional TF assay) were incubated with 180 μL of HEPES buffer containing 5.8 nmol/L purified human factor IXa (Alexis), 0.25 nmol/L purified human factor VIII (Alexis), and (where indicated) recombinant annexin V at increasing concentrations from 0.1 to 1,000 nmol/L for 5 minutes at 37°C. The reaction was then started by the addition of 20 μL of purified human factor X at a final concentration of 170 nmol/L. At various time points, 25 μL of the mixture was removed and factor Xa formation was monitored over 30 minutes, as described above. In samples containing annexin V, factor Xa was determined only in the plateau phase at 30 minutes. The amount of factor Xa was calculated as described above.
TM Activity
HUVECs (prepared as in the functional TF assay) were incubated with 200 μL of HEPES buffer containing 80 nmol/L purified human protein C (Sigma) and 0.05 U/mL human thrombin (Sigma) at 37°C. At various time points, 25 μL of the mixture was removed and the residual thrombin activity was quenched by the addition of 5 μL of hirudin at a final concentration of 0.25 U/mL. After 10 minutes, activated protein C (APC) was determined by the chromogenic substrate S-2366 (Chromogenix) at a final concentration of 0.2 mmol/L. The amount of APC was calculated by comparison with a standard curve made with known amounts of APC (Sigma). As a negative control, HUVECs were pretreated with 100 ng/mL LPS for 6 hours.
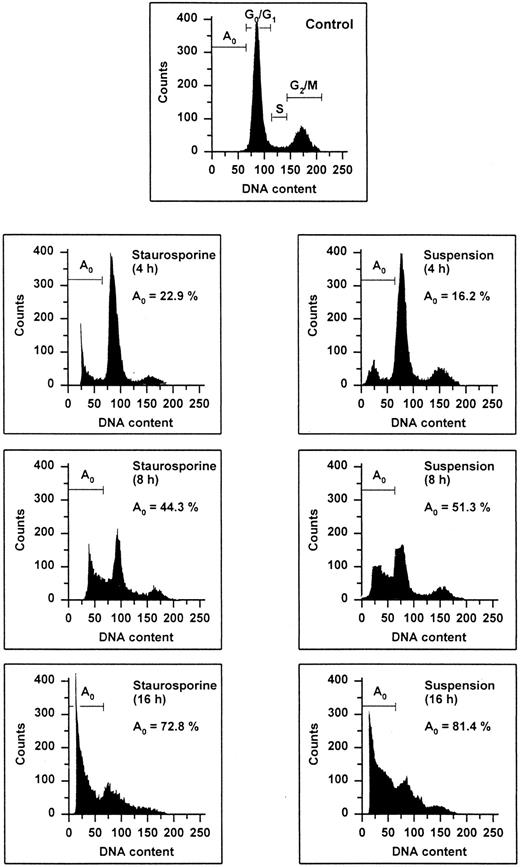
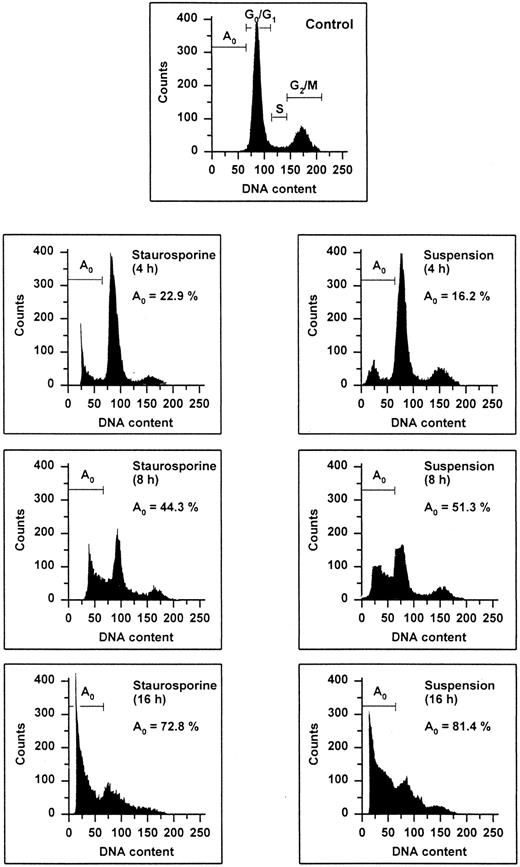
Flow cytometric cell cycle histogram. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 4, 8, and 16 hours were permeabilized, fixed, treated with RNAse, and stained with PI. The region below the Go /G1 peak (designated Ao region) represents cells undergoing apoptosis-associated DNA degradation (hypodiploid DNA) and is expressed as a percentage of events with respect to the entire cell cycle.
Flow cytometric cell cycle histogram. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 4, 8, and 16 hours were permeabilized, fixed, treated with RNAse, and stained with PI. The region below the Go /G1 peak (designated Ao region) represents cells undergoing apoptosis-associated DNA degradation (hypodiploid DNA) and is expressed as a percentage of events with respect to the entire cell cycle.
HS Activity
HUVECs (prepared as in the functional TF assay) were incubated with 190 μL of HEPES buffer containing 260 nmol/L purified human ATIII (Alexis) for 3 minutes. The reaction was started by the addition of 10 μL of human thrombin at a final concentration of 0.1 U/mL. At various time points, 25 μL of the mixture was removed and the residual thrombin activity was determined using 0.2 mmol/L chromogenic substrate S-2366. The amount of thrombin was calculated by comparison with a standard curve made with known amounts of thrombin. As a negative control, HUVECs were pretreated with 10 U/mL heparinase III (Sigma) for 6 hours.
TFPI Activity
TFPI activity of HUVECs was determined using a modified two-stage chromogenic assay as previously described.26 Briefly, HUVECs (prepared as in the functional TF assay) were incubated with 150 μL of HEPES buffer (with 20 mmol/L CaCl2 ) containing 1 nmol/L purified human factor VIIa (Alexis), 0.05 U/mL purified human factor Xa (Sigma), and 1:80 diluted rabbit brain thromboplastin (T-0263; Sigma) for 10 minutes at 37°C. The reaction was started by the addition of 50 μL of purified human factor X at a final concentration of 125 nmol/L. By removing 25 μL of the mixture, the residual activity of factor Xa was monitored over 45 minutes, as described above. As a negative control, HUVECs were treated with 60 μg/mL rabbit antihuman TFPI antibody for 1 hour at 37°C. The inhibitory effect of this antibody has previously been shown.27
Collagen Exposure
The determination of collagen exposure of adherent apoptotic HUVECs was performed as previously described.28 Briefly, HUVECs grown in collagen-coated (type IV, C-7521; Sigma) 48-well plates were blocked by PBS buffer, pH 7.8, with 10% bovine serum albumin for 30 minutes at 37°C. The cells were then incubated with 10 μg/mL biotinylated anticollagen MoAb (type IV, clone Col-94; Sigma) for 2 hours at 37°C. After harvesting the cells with 0.2 mg/mL EDTA, 5 μg/mL streptavidine alkaline phosphatase (Sigma) was added. After 1 hour, the reaction was started by the addition of 1 mg/mL p-nitrophenylphosphate (Sigma). The optical densities were measured at 405 nm after 1 hour. Between each step, the wells were washed twice.
Quantification of Cell Detachment
Staurosporine-treated HUVECs cultivated in 6-well plates were checked for the presence of spontaneously detached cells. Media (3 mL) were carefully removed and added to 1 mL 4% paraformaldehyde (Fluka, Buchs, Switzerland) to immediately fix the cells. The cells were then counted three times using a cell counter (Coulter Electronics Ltd, Luton, UK).
Thrombin Determination in Recalcified Plasma
Blood drawn from drug free healthy volunteers was anticoagulated by the addition of 1 part 110 mmol/L sodium citrate to 9 parts whole blood. Platelet-rich and platelet-poor plasma was prepared by centrifugation at 700g for 4 minutes and 2,000g for 30 minutes, respectively. Whereas staurosporine-treated HUVECs in a 6-well plate were incubated with 0.5 mL of platelet-rich plasma, HUVECs derived from culture in suspension were incubated with 0.5 mL of platelet-poor plasma. The coagulation process was started by the addition of 0.5 mL of 20 mmol/L CaCl2 (37°C). At various time points, 50 μL of recalcified plasma was removed and added to 50 μL of PBS buffer containing 20 mmol/L EDTA to stop thrombin formation. Thrombin activity was then determined by the chromogenic substrate S-2366 (final concentration, 0.2 mmol/L). The amount of thrombin was calculated as described above.
RESULTS
Apoptosis-Induced DNA Fragmentation
The occurrence of DNA fragmentation, as a feature of apoptosis, was determined by flow cytometric measurement of the percentage of nuclei with a hypodiploid DNA content, as previously described.29,30 This procedure has been shown to have an excellent correlation with colorimetric and electrophoretic methods for the detection of DNA fragments.29 As shown in Fig 1, PI staining of permeabilized normal HUVECs showed a typical distribution of cells at different stages in the cell cycle, as represented by a major diploid peak (Go /G1 ), a small hyperdiploid region (S), and a minor tetraploid peak (G2 /M). HUVECs in the region below the Go /G1 peak, designated Ao region, were defined as cells undergoing apoptosis-associated DNA degradation. Both staurosporine treatment and serum deprivation with culture in suspension led to a time-dependent increase of the percentage of hypodiploid cells. Within the first 4 hours, hypodiploid cells were principally recruited from cells in the S- and G2 /M-phase, whereas cells from the Go /G1 peak turned apoptotic only after 8 hours. At 16 hours, about 75% of the cells were found in the Ao region. Apoptosis of HUVECs was also confirmed by electrophoretic determination of internucleosomal DNA fragmentation (Fig 2). Thus, these data show that both methods, staurosporine-treatment and culture in suspension with serum-deprivation, induce efficiently apoptotic cell death in HUVECs.
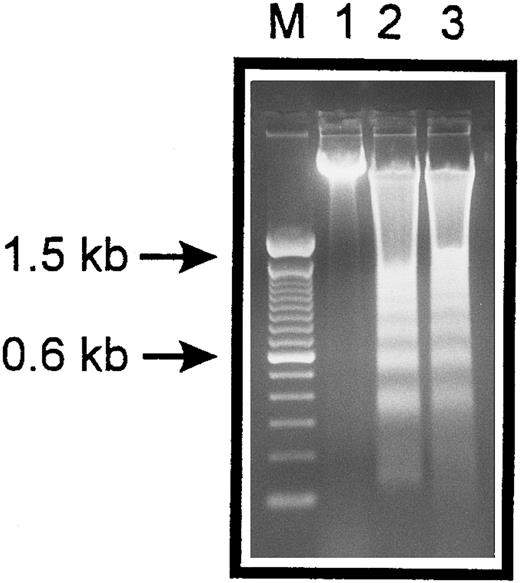
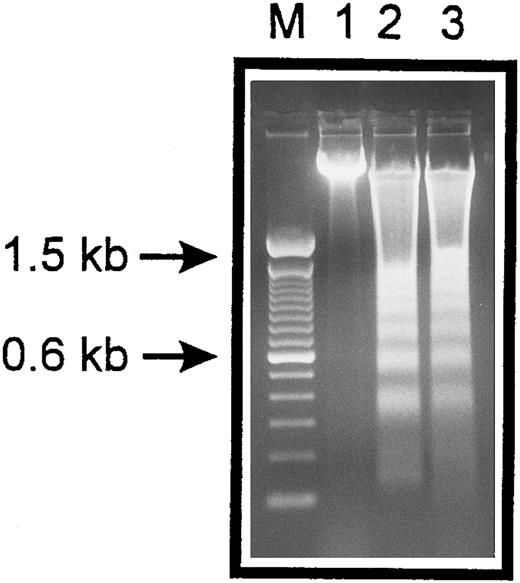
Internucleosomal DNA fragmentation. HUVECs treated with staurosporine (lane 2) or kept in suspension with serum deprivation (lane 3) for 6 hours were lysed and total cellular DNA was separated on a 2% agarose gel. HUVECs in lanes 2 and 3 show typical oligonucleosomal banding. Lanes M and 1 represent molecular weight markers (in kilobases) and normal HUVECs, respectively.
Internucleosomal DNA fragmentation. HUVECs treated with staurosporine (lane 2) or kept in suspension with serum deprivation (lane 3) for 6 hours were lysed and total cellular DNA was separated on a 2% agarose gel. HUVECs in lanes 2 and 3 show typical oligonucleosomal banding. Lanes M and 1 represent molecular weight markers (in kilobases) and normal HUVECs, respectively.
Apoptosis-Associated Annexin V Binding
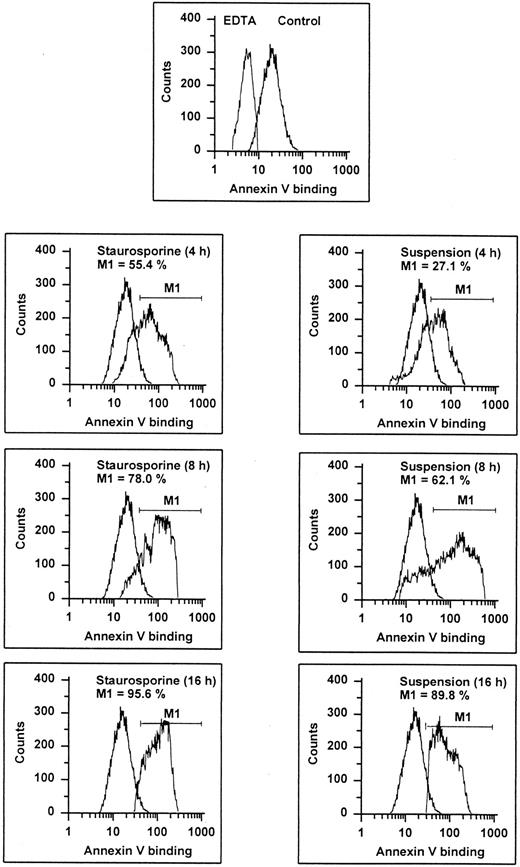
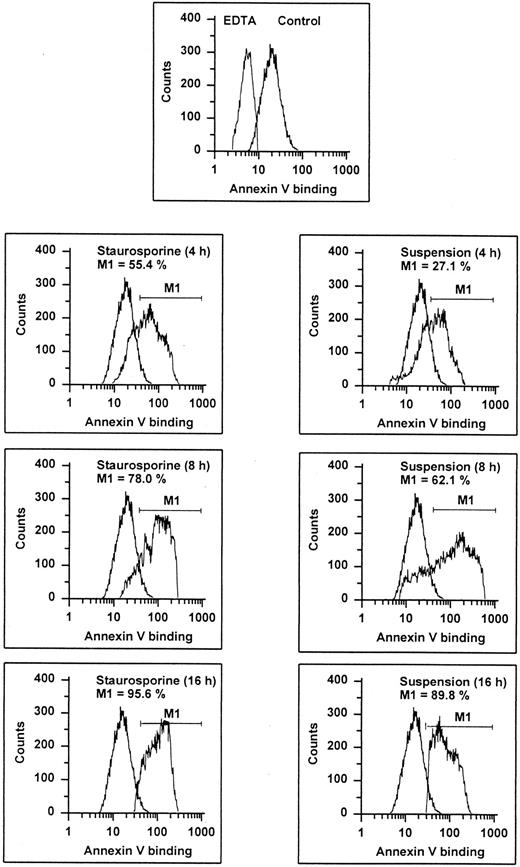
It is known that entry into apoptosis leads to a rapid loss of phospholipid asymmetry, with the subsequent exposure of PS on the outer leaflet.31 This early redistribution of PS has recently been found to occur independent of the initiating apoptotic stimulus.32 Based on the calcium-dependent high affinity of annexin V for negatively charged phospholipids, detection of PS expression can be performed by flow cytometric determination of annexin V binding, as shown in previous studies.32-34 Compared with negative controls (binding procedure in EDTA), almost all cells showed an enhanced fluorescence indicating that even unperturbed endothelial cells may expose PS (Fig 3). However, both staurosporine treatment and culture in suspension led to a further increase of annexin V binding. At 16 hours, about 90% of the cells displayed an enhanced fluorescence. To exclude increased annexin V binding due to necrosis, staurosporine-treated cells were tested for propidium iodide uptake without permeabilization. At 8 hours, less than 15% of the cells were positive for both PI and annexin V (data not shown), indicating that enhanced exposure of negatively charged phospholipids is primarily caused by apoptosis.
Annexin V binding. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 4, 8, and 16 hours were stained with FITC-conjugated recombinant annexin V in a buffer containing 2.5 mmol/L CaCl2 and subsequently analyzed using flow cytometry. Almost 100% of normal cells (upper panel, right peak) show an increased fluorescence compared with cells stained in EDTA. The marker M1 represents the percentage of cells exhibiting a higher fluorescence than the control (lower 6 panels, left peak). The results of one representative experiment of three performed are shown.
Annexin V binding. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 4, 8, and 16 hours were stained with FITC-conjugated recombinant annexin V in a buffer containing 2.5 mmol/L CaCl2 and subsequently analyzed using flow cytometry. Almost 100% of normal cells (upper panel, right peak) show an increased fluorescence compared with cells stained in EDTA. The marker M1 represents the percentage of cells exhibiting a higher fluorescence than the control (lower 6 panels, left peak). The results of one representative experiment of three performed are shown.
TF Expression
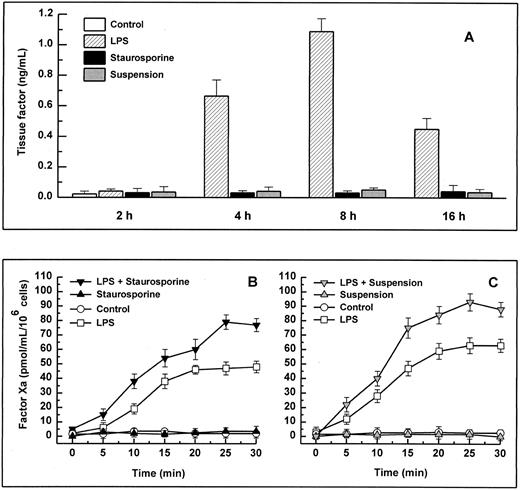
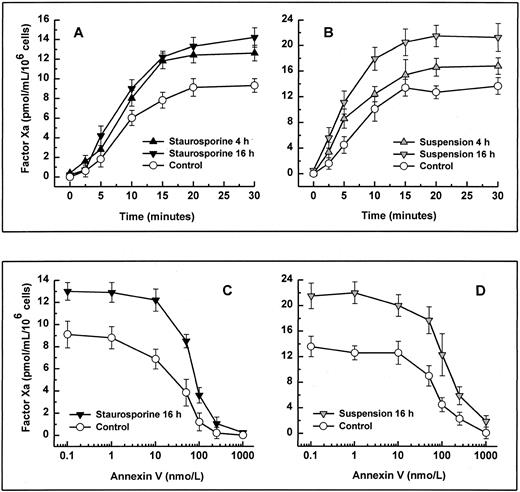
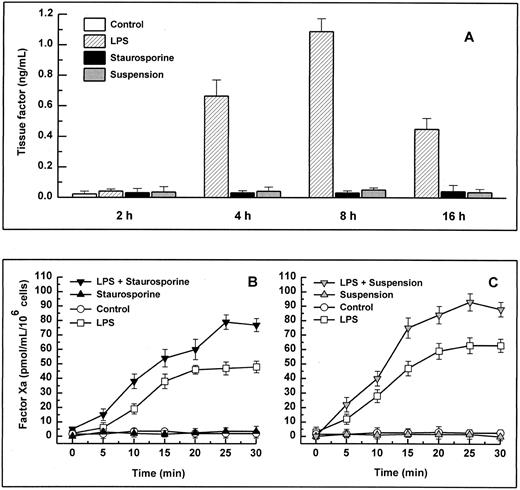
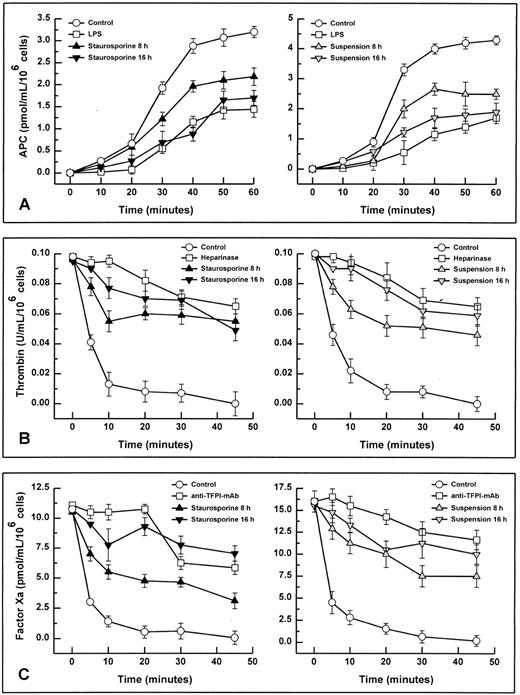
When stimulated by different inflammatory cytokines, cultured endothelial cells are well known to rapidly express TF, leading to the initiation of the extrinsic coagulation system.5 Because apoptosis may require protein synthesis, apoptotic HUVECs were probed for TF expression. Measured over a time period from 2 to 16 hours, HUVECs treated with staurosporine or culture in suspension with serum starvation did not show increased antigenic TF levels or enhanced functional activity (Fig 4A, B, and C). However, when activated with LPS, formation of factor Xa was increased by about 50% to 70% on apoptotic cells compared with live cells (Fig 4B and C). Thus, apoptosis seems to further enhance the functional activity of TF that is already expressed on the cell surface rather than induce its de novo synthesis.
Antigenic TF. (A) HUVECs were treated with LPS (▨) or staurosporine (▪) or were kept in suspension with serum deprivation () for 2, 4, 8, and 16 hours. Cells were then lysed and prepared for quantitative determination of TF by ELISA. Functional TF in adherent (B) and suspended cells (C). HUVECs either untreated or activated with LPS for 6 hours were treated with staurosporine (B; solid triangles) or kept in suspension with serum deprivation (C; shaded triangles) for 8 hours. After incubation with factor VIIa and factor X, the formation of factor Xa was measured using a chromogenic substrate. Controls were untreated cells (B and C; ○) and cells treated with LPS (B and C; □). Results are expressed as means ± SD (n = 4).
Antigenic TF. (A) HUVECs were treated with LPS (▨) or staurosporine (▪) or were kept in suspension with serum deprivation () for 2, 4, 8, and 16 hours. Cells were then lysed and prepared for quantitative determination of TF by ELISA. Functional TF in adherent (B) and suspended cells (C). HUVECs either untreated or activated with LPS for 6 hours were treated with staurosporine (B; solid triangles) or kept in suspension with serum deprivation (C; shaded triangles) for 8 hours. After incubation with factor VIIa and factor X, the formation of factor Xa was measured using a chromogenic substrate. Controls were untreated cells (B and C; ○) and cells treated with LPS (B and C; □). Results are expressed as means ± SD (n = 4).
Intrinsic Tenase Complex
Binding and activation of coagulation factors on platelets are known to occur much more efficiently upon platelet activation.35,36 This enhanced procoagulant activity is caused by the exposure of anionic phospholipids, as shown by an increased binding of annexin V.37 To determine whether the rapid loss of the membrane phospholipid asymmetry in apoptotic endothelial cells, as seen in Fig 3, would also increase the activity of coagulation factors, we measured the activity of the reconstituted intrinsic tenase complex. Independent of the method of apoptosis induction, formation of factor Xa by factor IXa and VIII was increased significantly on HUVECs undergoing cell death. Enhanced exposure of PS of 27.1%, as seen in suspended cells for 4 hours (Fig 3), already led to an increased tenase activity of about 25% (Fig 5B). Formation of factor Xa further increased by about 50% to 60% using cells that were apoptotic for 8 hours (data not shown). Although maximal PS exposure was reached at 16 hours (Fig 3), intrinsic tenase activity did not further enhance (Fig 5A and B). To decide whether this increased activity was caused by enhanced PS exposure, recombinant annexin V was probed as a competitive inhibitor. Coincubation of annexin V with factor IXa and VIII dose-dependently inhibited generation of factor Xa on both normal and apoptotic HUVEC (Fig 5C and D). The concentration of annexin V causing 50% inhibition of tenase activity was 41 ± 12 nmol/L and 78 ± 21 nmol/L for adherent normal and apoptotic cells, respectively. The corresponding values of cells in suspension were 62 ± 24 nmol/L and 104 ± 38 nmol/L, respectively. Hence, increased annexin V binding in apoptotic HUVECs correlates with intrinsic tenase activity, and the extent to which annexin V binds to apoptotic HUVECs correlates with its ability to inhibit the intrinsic tenase activity.
Activity and inhibition of the intrinsic tenase complex. Assays were performed using either adherent (left panels) or suspended cells (right panels). Apoptosis was induced by treatment with staurosporine (left panels, solid triangles) or culture in suspension with serum deprivation (right panels, shaded triangles) for 4 and 16 hours, respectively. The activity of the intrinsic tenase complex was determined by measuring the amidolytic activity of factor Xa after the addition of factor IXa, factor VIII, and factor X (A and B). The effect of the exposure of PS on the activity of the intrinsic tenase complex was assessed by the ability of annexin V to inhibit factor Xa formation (C and D). After preincubation of HUVECs with various concentrations of annexin V for 5 minutes, the activity of the intrinsic tenase complex was measured as described above. Controls were untreated cells (all panels; ○). Results are expressed as means ± SD (n = 4).
Activity and inhibition of the intrinsic tenase complex. Assays were performed using either adherent (left panels) or suspended cells (right panels). Apoptosis was induced by treatment with staurosporine (left panels, solid triangles) or culture in suspension with serum deprivation (right panels, shaded triangles) for 4 and 16 hours, respectively. The activity of the intrinsic tenase complex was determined by measuring the amidolytic activity of factor Xa after the addition of factor IXa, factor VIII, and factor X (A and B). The effect of the exposure of PS on the activity of the intrinsic tenase complex was assessed by the ability of annexin V to inhibit factor Xa formation (C and D). After preincubation of HUVECs with various concentrations of annexin V for 5 minutes, the activity of the intrinsic tenase complex was measured as described above. Controls were untreated cells (all panels; ○). Results are expressed as means ± SD (n = 4).
TM Expression
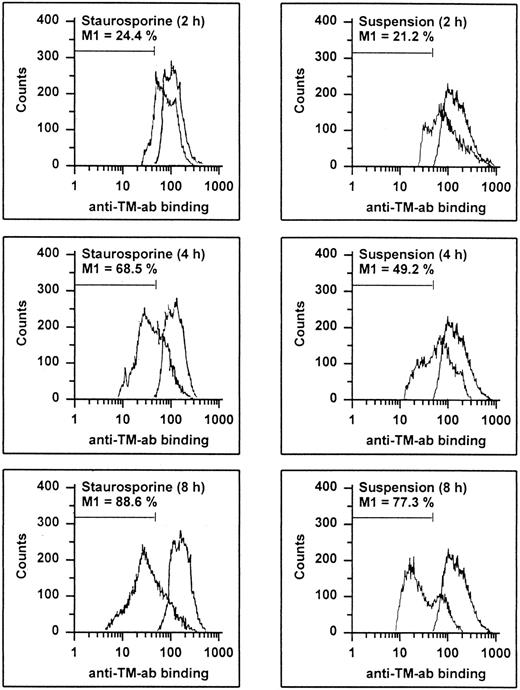
HUVECs undergoing apoptosis showed a time-dependent decrease of antigenic TM expression (Fig 6). At 8 hours, 88.6% and 77.3% of the cells treated with staurosporine or cultured in suspension with serum deprivation displayed a reduced fluorescence, respectively. This result was more than the maximal decrease observed with HUVECs treated with LPS (about 46%), a stimulus known to induce TM downregulation.38 Accordingly, TM activity of apoptotic HUVECs, as measured as thrombin-dependent activation of protein C, decreased in a time-dependent manner as well (Fig 7A). However, after 8 and 16 hours, there was only a reduction of about 32% and 47% for staurosporine-treated cells and 40% and 57% for cells in suspension, respectively. This decrease after 16 hours was about in the range of LPS-treated cells. Because both assays (adherent and suspended cells) showed similar results, a decrease of TM activity caused by staurosporine-induced reduction of cell surface (retraction and detachment) could be excluded.
Flow cytometric determination of TM. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 2, 4, and 8 hours were harvested, washed, and stained with an antihuman TM MoAb, followed by an FITC-conjugated goat antimouse antibody. The M1-region is defined as a percentage of events below the control (all panels, right peak). The results of one representative experiment of three performed are shown.
Flow cytometric determination of TM. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 2, 4, and 8 hours were harvested, washed, and stained with an antihuman TM MoAb, followed by an FITC-conjugated goat antimouse antibody. The M1-region is defined as a percentage of events below the control (all panels, right peak). The results of one representative experiment of three performed are shown.
Functional activity of TM (A), HS (B), and TFPI (C). Assays were performed using either adherent (left panels) or suspended cells (right panels). Apoptosis in adherent and suspended HUVECs was induced by treatment with staurosporine (left panels, solid triangles) or culture in suspension with serum deprivation (right panels, shaded triangles) for 8 and 16 hours, respectively. All activities were determined by chromogenic substrate assays as follows: thrombin-dependent activation of protein C (TM activity), ATIII-dependent inhibition of thrombin (HS activity), and inhibition of factor Xa formation generated by the TF-factor VIIa complex (TFPI activity). Controls were untreated cells (all panels; ○). As negative controls (all panels; □), cells were pretreated with LPS (TM), heparinase III (HS), and rabbit antihuman TFPI antibody (TFPI), respectively. Results are expressed as mean ± SD (n = 4).
Functional activity of TM (A), HS (B), and TFPI (C). Assays were performed using either adherent (left panels) or suspended cells (right panels). Apoptosis in adherent and suspended HUVECs was induced by treatment with staurosporine (left panels, solid triangles) or culture in suspension with serum deprivation (right panels, shaded triangles) for 8 and 16 hours, respectively. All activities were determined by chromogenic substrate assays as follows: thrombin-dependent activation of protein C (TM activity), ATIII-dependent inhibition of thrombin (HS activity), and inhibition of factor Xa formation generated by the TF-factor VIIa complex (TFPI activity). Controls were untreated cells (all panels; ○). As negative controls (all panels; □), cells were pretreated with LPS (TM), heparinase III (HS), and rabbit antihuman TFPI antibody (TFPI), respectively. Results are expressed as mean ± SD (n = 4).
HS Expression
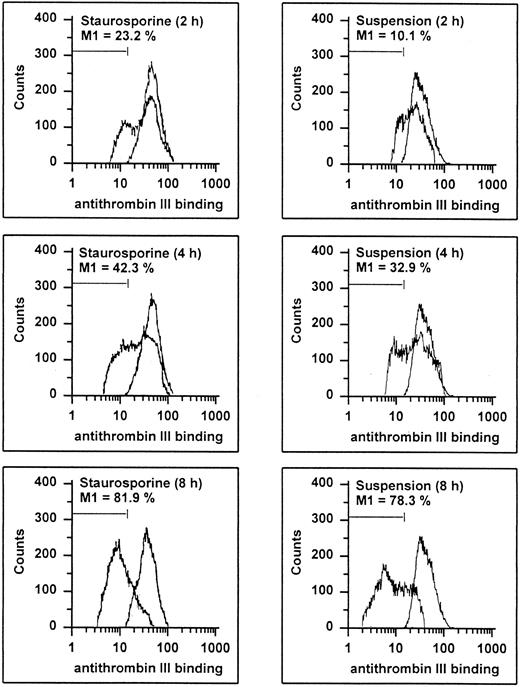
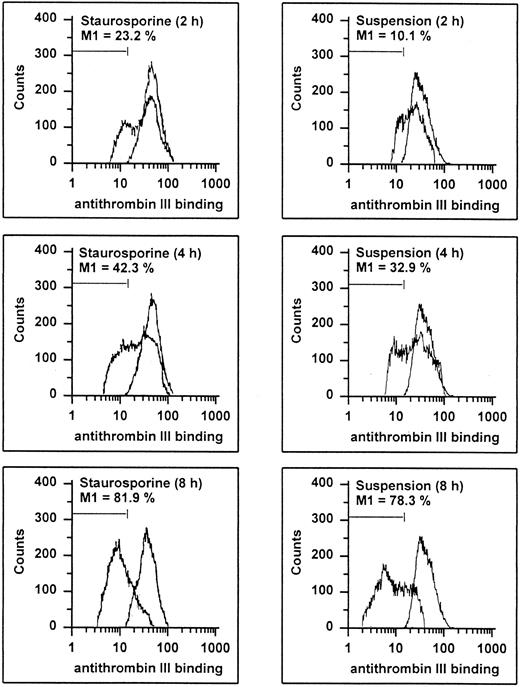
Endothelial cells synthesize different types of proteoglycans that contain HS chains capable of binding and activating ATIII to accelerate thrombin inhibition.2 39 Thus, the capacity of endothelial cells to bind ATIII corresponds to their content of anticoagulantly active HS. As shown in Fig 8, HUVECs undergoing apoptosis showed a time-dependent decrease in ATIII binding. At 8 hours, 81.9% and 78.3% of the cells treated with staurosporine or culture in suspension with serum deprivation exhibited reduced fluorescence. This loss of ATIII binding was very similar to that of cells treated with heparinase III displaying a reduction of about 90% after 6 hours. HS activity of apoptotic HUVECs, as determined as cell-mediated acceleration of thrombin inhibition by ATIII, also decreased in a time-dependent manner (Fig 7B). Independent of the method of apoptosis induction, thrombin inhibition of apoptotic cells was reduced by about 50% after 8 and 16 hours, which corresponded to cells treated with heparinase III. At 2 and 4 hours, there was no significant decrease (data not shown).
Flow cytometric determination of HS. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 2, 4, and 8 hours were harvested, washed, and incubated with ATIII for 30 minutes. Cells were then stained with an antihuman ATIII MoAb, followed by an FITC-conjugated goat antimouse antibody. The M1-region is defined as the percentage of events below the control (all panels, right peak). The results of one representative experiment of three performed are shown.
Flow cytometric determination of HS. HUVECs treated with staurosporine (left panels) or kept in suspension with serum deprivation (right panels) for 2, 4, and 8 hours were harvested, washed, and incubated with ATIII for 30 minutes. Cells were then stained with an antihuman ATIII MoAb, followed by an FITC-conjugated goat antimouse antibody. The M1-region is defined as the percentage of events below the control (all panels, right peak). The results of one representative experiment of three performed are shown.
TFPI Expression
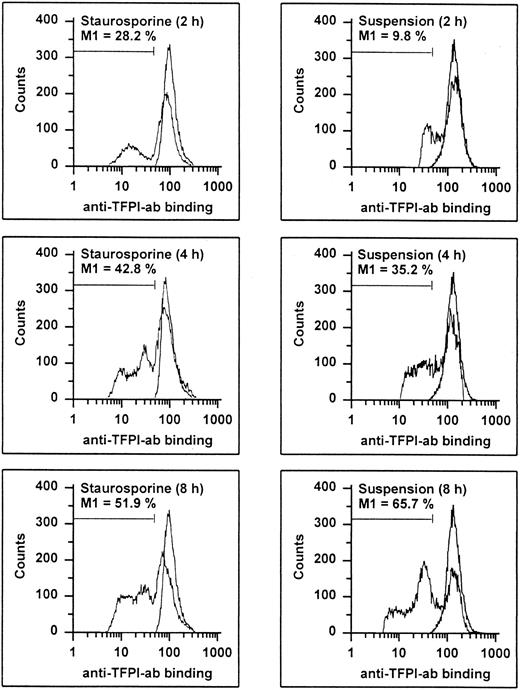
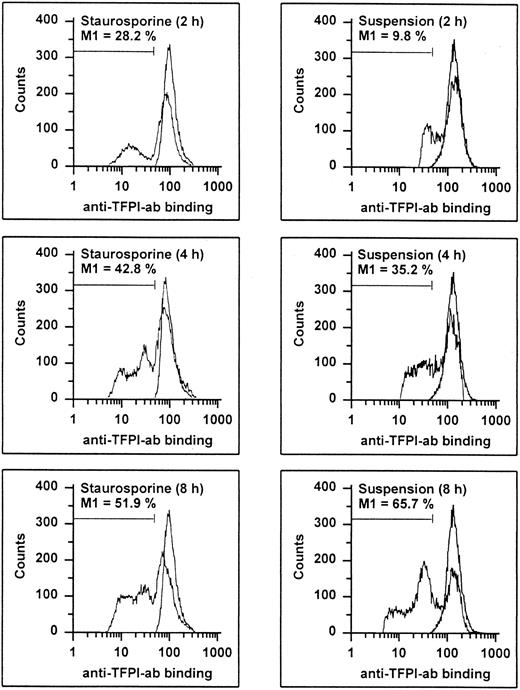
It has recently been shown that endothelial TFPI is stored intracellularly in specific granules and is also present on the surface, probably anchored to glycosaminoglycans.27 Permeabilized apoptotic HUVECs showed a time-dependent decrease of intracellular TFPI antigen levels (Fig 9). At 8 hours, 51.9% and 65.7% of the cells treated with staurosporine or culture in suspension with serum deprivation displayed reduced fluorescence, respectively. Antigenic TFPI levels of unpermeabilized HUVECs showed a similar decrease (data not shown). Also, independent of the method of apoptosis induction, the inhibitory activity of TFPI was found to be reduced by about 38% and 63% after 8 and 16 hours, respectively (Fig 7C). This reduction at 16 hours was similar to that obtained with cells treated with the inhibitory antihuman TFPI antibody. Hence, both intracellular and surface-bound TFPI contents were found to be affected during apoptosis.
Flow cytometric determination of TFPI. HUVECs treated with staurosporine (left panels) or kept in suspension with serum-deprivation (right panels) for 2, 4, and 8 hours were harvested, washed, and stained with a rabbit antihuman TFPI antibody, followed by an FITC-conjugated antirabbit MoAb. The M1-region is defined as the percentage of events below the control (all panels, right peak). The results of one representative experiment of three performed are shown.
Flow cytometric determination of TFPI. HUVECs treated with staurosporine (left panels) or kept in suspension with serum-deprivation (right panels) for 2, 4, and 8 hours were harvested, washed, and stained with a rabbit antihuman TFPI antibody, followed by an FITC-conjugated antirabbit MoAb. The M1-region is defined as the percentage of events below the control (all panels, right peak). The results of one representative experiment of three performed are shown.
Collagen Exposure
Normal HUVECs that were completely confluent by light microscopy exhibited less than 5% exposure of collagen, as determined by direct competitive ELISA. However, when treated with staurosporine, cells immediately began to retract, resulting in a time-dependent increase of exposure of the subendothelial matrix. Treatment of 2 hours (or only 30 minutes) led to a significant increase to 20% ± 2%. After 6, 8, and 16 hours, there were further increases to 32% ± 6%, 46% ± 7%, and 63% ± 6%, respectively. Because media derived from HUVECs treated with staurosporine for 0.5, 2, 4, and 6 hours did not contain more floating cells than untreated cells (detachment rate of about 2% on average), it was concluded that the increase of matrix exposure within the first 6 hours was caused only by cell retraction. However, at 8 and 16 hours, staurosporine-treated cells showed an enhanced detachment rate of 6.8% ± 4.1% and 20.3% ± 7%, respectively.
Thrombin Formation in Recalcified Plasma
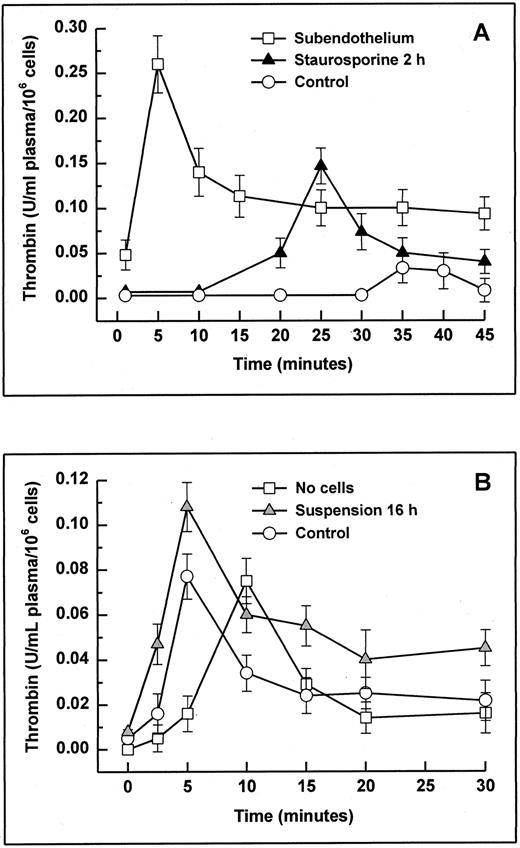
The potent anticoagulant properties of cultured endothelial cells have been shown most effectively in nonanticoagulated whole blood28,40,41 and recalcified plasma.28 42 To assess whether matrix exposure caused only retraction would induce a procoagulant effect, citrated platelet-rich plasma was recalcified on HUVECs treated with staurosporine for 2 hours, and the formation of thrombin was monitored (Fig 10A). The total amount of thrombin (integral) formed on staurosporine-treated cells was about 5 times higher than on normal HUVECs, and the maximal peak was about 10 minutes earlier. Results from cells treated for 4 and 6 hours did not differ (data not shown). On completely deendothelialized matrix, the total thrombin formation was about 10 times higher. Floating apoptotic HUVECs (induced by culture in suspension with serum deprivation for 16 hours) were tested using platelet-poor plasma to minimize a potential masking effect of activated platelets (Fig 10B). Likewise, apoptotic cells induced increased thrombin formation of almost 50% compared with normal HUVECs. It is noteworthy that, in contrast to adherent live cells, floating live cells also led to an accelerated generation of thrombin.
Thrombin generation in plasma with adherent (A) or suspended cells (B). Apoptosis in adherent and suspended HUVECs was induced by treatment with staurosporine for 2 hours (A; solid triangles) and by culture in suspension with serum deprivation for 16 hours (B; shaded triangles), respectively. Cells were then incubated with 0.5 mL of citrated platelet-rich (adherent cells) and platelet-poor plasma (suspended cells). After starting the coagulation process with 0.5 mL of prewarmed CaCl2 , the formation of thrombin was monitored using a chromogenic substrate. Controls were untreated cells (A and B; ○). As negative controls (A and B; □), subendothelial matrix and no cells were used, respectively. Results are expressed as mean ± SD (n = 6).
Thrombin generation in plasma with adherent (A) or suspended cells (B). Apoptosis in adherent and suspended HUVECs was induced by treatment with staurosporine for 2 hours (A; solid triangles) and by culture in suspension with serum deprivation for 16 hours (B; shaded triangles), respectively. Cells were then incubated with 0.5 mL of citrated platelet-rich (adherent cells) and platelet-poor plasma (suspended cells). After starting the coagulation process with 0.5 mL of prewarmed CaCl2 , the formation of thrombin was monitored using a chromogenic substrate. Controls were untreated cells (A and B; ○). As negative controls (A and B; □), subendothelial matrix and no cells were used, respectively. Results are expressed as mean ± SD (n = 6).
DISCUSSION
The integrity of the endothelium is crucial for the maintenance of the antithrombotic state. Perturbation or injury of endothelial cells leading to exposure of TF and extracellular matrix are well known to induce the coagulation process. However, the behavior of endothelial cells undergoing apoptosis is not known. In view of the increasing evidence of occurrence of vascular cell apoptosis in inflammatory and atherosclerotic lesions,8-10 we attempted to characterize the anticoagulant and procoagulant properties of endothelial cells during the process of cell death.
To induce apoptosis in adherent endothelial cells, we treated HUVECs with staurosporine, a nonspecific protein kinase inhibitor, that has frequently been used to induce apoptosis in other cells, such as thymocytes,43 different human ovarian and leukemia cell lines,32,44,45 central neurons,46 and fibroblasts.47 Because these studies have used different staurosporine concentrations (from 1 to 2,500 nmol/L), it is difficult to decide whether HUVECs are more or less susceptible to staurosporine to undergo chromatin cleavage. However, except for MOLT-4 cells,44 DNA fragmentation was found to be completed in most cells within 16 to 24 hours, which is comparable to our results. To test floating apoptotic cells and to verify that the results obtained with staurosporine would indeed represent effects caused by apoptosis rather than by staurosporine itself, we additionally induced cell death by culture in suspension and serum deprivation. Both have individually been shown to induce efficiently apoptosis in endothelial cells within 24 to 30 hours.22 Thus, the simultaneous occurrence of oligonucleosomal fragments at 6 hours and 70% to 80% of hypodiploid cells at 16 hours using either staurosporine or culture in suspension with serum deprivation indicates that both methods show analogous efficiency to induce apoptotic cell death in HUVECs.
Changes in phospholipid asymmetry with exposure of PS is another fundamental feature of apoptosis.31 Based on the high affinity for aminophospholipids, annexin V has been found to provide a convenient tool to determine kinetics of PS exposure.32-34 In accordance with previous findings showing that redistribution of plasma membrane PS appears several hours earlier than nuclear changes,32 HUVECs were found to display increased annexin V binding before DNA fragmentation independent of the method of apoptosis induction. This may also be due to preexistent annexin V binding sites on normal HUVECs, as shown by a reduced fluorescence of normal cells in the presence of EDTA and also shown in previous studies.4
On activated platelets, exposure of PS on the outer leaflet is essential for efficient activation of coagulation factors.37-39 Negatively charged phospholipids are similarly involved in factor VIII- and factor IXa-dependent activation of factor X on endothelial cells, because annexin V is known to completely inhibit this reaction.4 However, activation of endothelial cells by different cytokines and phorbol esters does not further increase the binding sites for annexin V4 or the activity of the intrinsic tenase complex.48 However, upon apoptotic cell death, we found that endothelial cells are able to enhance both the exposure of PS and, concomitantly, the rate of activation of factor X by factor VIII and factor IX. Accordingly, in a monocytic cell line undergoing apoptotic cell death, enhanced exposure of anionic phospholipids has also been found to induce an increased activity of the prothrombinase complex.49 Hence, apoptosis seems to induce a procoagulant behavior in a platelet-like manner. If significant endothelial apoptosis occurs in atherosclerotic lesions, this observation may explain, in part, two recent reports showing increased prothrombin activation on aortic segments obtained from hypercholesterinemic rabbits50 and on endothelial cells isolated from areas of human aortas at high risk for atherosclerosis.51
Based on responses of endothelial cells in vitro, TF is postulated to be expressed by activated endothelium at sites of inflammation. However, TF synthesis seems not to be initiated during endothelial cell death, because we did not find an increase of antigen levels or functional activity. However, when TF is expressed on the cell surface upon preactivation with LPS, apoptotic cells significantly increase factor VIIa-mediated formation of factor Xa. This observation confirms previous studies showing that endotoxin-stimulated endothelial cells and TF expressing monocytic cells display enhanced TF procoagulant activity upon induction of apoptosis.49,52 It has been suggested that this effect may be caused by de-encryption of TF, as it has been found upon cell lysis,53 and enhanced binding and activation of coagulation factors due to exposure of negatively charged phospholipids.
Both TM and HS are well known to be rapidly downregulated upon perturbing agents, such as inflammatory cytokines, endotoxins, hypoxia, and homocysteinemia.1,2,40 In view of the fact that some of these substances are also able to induce apoptosis in endothelial cells,14-16 the rapid loss of TM and HS observed during apoptotic cell death is not surprising. We cannot determine whether this loss is caused by cleavage or altered protein synthesis, because we did not measure these molecules in the supernatants. However, regarding the rapidity of disappearance and the fact that soluble TM and HS in plasma are thought to be markers of endothelial cell injury, we suspect a release reaction. In contrast, loss of TFPI occurred more slowly, which may indicate another mechanism of downregulation. Because we found that thrombin, which is known to induce an acute release of TFPI,27 leads to increased TFPI activity and antigen levels (unpublished observations), loss of TFPI in apoptotic HUVECs seems consequently more likely to be due to altered protein synthesis than to release. In neutrophils and myelomonocytic cells, not all surface glycoproteins have been found to be susceptible to undergo apoptosis-associated downregulation, suggesting that generalized loss of surface molecules does not occur during cell death.54 55 Thus, it is conceivable that other endothelial surface proteins might not be altered during apoptosis and that TM and HS are more susceptible than other surface molecules.
Because, as a general feature of apoptosis, cells rapidly retract, round up, and subsequently lose their intercellular contacts,56 it was assumed that apoptotic HUVECs may expose substantial areas of the highly procoagulant subendothelial matrix. Indeed, even without cell detachment, HUVECs increased collagen exposure from 5% to 30% within the first 6 hours of cell death, thereby inducing dramatic enhancement of thrombin formation in recalcified plasma. This procoagulant effect is analogous to a previous study showing that the anticoagulant effect of endothelial cells in whole blood or plasma is overcome by exposure of collagen by about 5% to 10% in dependence on the presence of platelets.28 Because retraction of endothelial cells upon exposure to inflammatory cytokines has been shown to involve protein kinase C,57 it is possible that staurosporine might influence the retraction process. However, staurosporine rather inhibits cell retraction or induces cell spreading.58,59 Thus, it is likely that an area of apoptotic endothelial cells in vivo may lead to significant exposure of subendothelial matrix. The significance of enhanced PS exposure and loss of anticoagulant molecules in plasma seems to be reflected in the observation that floating apoptotic HUVECs were also found to increase thrombin formation in plasma. Using a modified Russel viper venom test, a recent study has similarly shown that apoptotic leukocytic and endothelial cells exert a procoagulant effect in plasma.60 Thus, it is tempting to speculate that detached endothelial cells may lose immediately their anticoagulant properties and circulate as procoagulant bodies in a platelet-like manner.
ACKNOWLEDGMENT
The authors thank T. Eunson for preparing cell cultures and Dr D. Coder for assistance with flow cytometry.
Supported by US Public Health Service Grants No. HL 47151 and HL 18645. T.B. is the recipient of a grant of the Swiss National Science Foundation. A.K. is the recipient of a Clinician-Scientist Award of the Medical Research Council of Canada.
Address reprint requests to Thomas Bombeli, MD, Division of Hematology, Box 357710, University of Washington, Seattle, WA 98195.