To the Editor:
Over the past 20 years, efforts from several laboratories have led to the identification and synthesis of chromogenic substrates specific for thrombin, factor Xa, trypsin, kallikreins and urokinase, plasmin and tissue plasminogen activator, and other enzymes.1 However, several important proteases remain in need of specific chromogenic substrates and are difficult to characterize functionally. Perhaps the most notable example is offered by the vitamin K-dependent anticoagulant factor activated protein C (APC). This protease cleaves and inactivates factors Va and VIIIa and also stimulates the release of the fibrinolytic factor tissue plasminogen activator.2 Resistance to APC caused by a single amino acid substitution in factor Va leads to a serious thrombotic diathesis that affects 5% of the population.3 For this reason, APC is routinely tested in clinical laboratory analysis. Current tests of APC involve human plasma, which contains other blood clotting proteases such as thrombin and factor Xa.4 Similar tests are used in quantitative studies of protein C hydrolysis by thrombin, in which progress curves of chromogenic substrate hydrolysis are measured to reconstruct the time course of APC generation.5 In both instances, accuracy and sensitivity of the assay depend strongly on the specificity and selectivity of the chromogenic substrate used for testing APC and will obviously benefit from substrates highly selective for this protease.
A number of substrates have been reported to be specific for APC,6 but they lack selectivity in so far as they interact quite well, if not better, with thrombin. For example, S2366 (pyroGlu-Pro-Arg-p-nitroanilide) is the most specific substrate for APC, but unfortunately is even more specific for thrombin and cannot be used in clinical laboratory tests.4 S2266 (H-D-Val-Leu-Arg-p-nitroanilide) is slightly less specific than S2366, but has the desirable advantage of a comparable specificity for APC and thrombin, which currently makes it the substrate of choice in laboratory tests.4 5
Butenas et al7,8 have synthesized a number of fluorogenic substrates and shown that the presence of Arg at P1 and P2 results in increased selectivity toward APC. Also, the presence of bulky hydrophobic residues at P3 enhances specificity toward APC.6,7 By inspecting the sites of cleavage for APC in the natural substrate factor Va, we noticed the sequences Trp-Arg-Arg and Asp-Arg-Arg.9 Both sequences have an Arg residue at P2, which would certainly cause severe steric hindrance at the S2 site of thrombin, and one sequence also bears a bulky aromatic group at P3. Based on this observation and the previous work performed on fluorogenic substrates, we synthesized four chromogenic substrates using the two sequences cut in factor Va and using the two possible enantiomers for the residue at P3 in view of the well documented preference of thrombin for the D-enantiomer of aromatic/hydrophobic residues at P3.10 Chromogenic substrates are much more desirable for practical applications compared with the fluorogenic analogs, because they are easier to synthesize in large quantities and simplify data collection in routine clinical and laboratory tests.
The specificity constants s = kcat /Km for the hydrolysis of these substrates by thrombin, APC, and factor Xa are summarized in Table 1. The chromogenic substrate S2266 is included in the analysis for the sake of comparison. The new substrates have similar specificity for factor Xa, suggesting that the residue at P3 does not contact the active site of the enzyme. In the case of thrombin and APC, on the other hand, the group at P3 is crucial to determine both specificity and selectivity. This result shows that the presence of Arg at P1 and P2 is necessary but not sufficient to gain selectivity toward APC, contrary to the expectations derived from previous studies with fluorogenic substrates.7 Asp at P3 is recognized more effectively by thrombin in the L-enantiomer, as found in the natural substrates protein C and the thrombin receptor, whereas the D-enantiomer causes a loss of specificity of more than 1,600-fold due to a decrease in the kcat (Table 2). The side chain of H-D-Asp would make a very unfavorable interaction with the aromatic moiety lining the S3 specificity site of thrombin,10 whereas the side chain of H-L-Asp may be oriented favorably to couple electrostatically with the side chain of Arg at P2, thereby reducing the unfavorable interaction of this residue with the S2 site. When Trp is present at P3, thrombin prefers the D-enantiomer, consistent with the side chain of Trp making a favorable interaction with W215 in the D-, but not the L-enantiomer.10 In the case of APC, the enantiomer of Asp at P3 is inconsequential, although the similar specificity of the two enantiomers results from very different values of kcat and Km (Table 2). Trp at P3 is strongly preferred in the D-enantiomer, which has a significantly higher kcat .
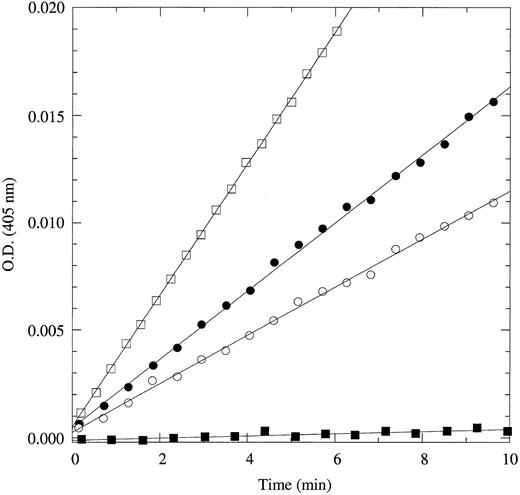
Of the four new substrates, H-D-Trp-Arg-Arg-p-nitroanilide is the most specific and selective for APC. Compared with Gly-Gly-Arg-Arg-ANSNH(cyclo-C6H11 ), the most selective fluorogenic substrate currently available for APC,7 H-D-Trp-Arg-Arg-p-nitroanilide is sixfold more specific and 1.2-fold more selective over thrombin. Compared with S2266, H-D-Trp-Arg-Arg-p-nitroanilide is 1.4-fold more specific and 100-fold more selective over thrombin. H-D-Trp-Arg-Arg-p-nitroanilide should become the substrate of choice in quantitative assays of APC and protein C cleavage by thrombin. This point is illustrated convincingly by the data in Fig 1. The fraction of substrate cleaved by thrombin in a solution containing 1 nmol/L each of APC and thrombin is at most an insignificant 3%. The absorbance signal generated by the substrate cleaved by thrombin over 10 minutes is also indistinguishable from noise (±0.0001 OD units) even when using an instrument as accurate as a Cary3 dual-beam spectrophotometer. In the same experiment performed with S2266, the amount of substrate cleaved by thrombin over the same time scale is threefold the amount cleaved by APC. Furthermore, a similar assay using 100 μmol/L H-D-Trp-Arg-Arg-p-nitroanilide can accurately detect APC at concentrations as low as 100 pmol/L in as little as 15 minutes. A final remark should be made on the lower value of Km for this substrate (84 μmol/L) compared with S2266 (210 μmol/L) or Gly-Gly-Arg-Arg-ANSNH(cyclo-C6H11 ) (267 μmol/L), which will significantly reduce both materials and costs for laboratory tests.
Hydrolysis of 60 μmol/L H-D-Trp-Arg-Arg-p-nitroanilide (•, ▪) or S2266 (○, □) by 1 nmol/L human APC (•, ○) or human thrombin (▪, □). Data points depict the absorbance at 405 nm due to the released p-nitroaniline (ε405 = 9,920 mol/L−1cm−1) from the cleaved substrate. Experimental conditions are 5 mmol/L Tris, 0.1% PEG, 200 mmol/L NaCl, pH 8.0, at 25°C. When using H-D-Trp-Arg-Arg-p-nitroanilide, APC at a concentration of 1 nmol/L can easily be detected and gives an absorbance signal of released p-nitroaniline after 10 minutes that is 160-fold the noise level of ±0.0001 OD units (or ±10 nmol/L p-nitroaniline) of a Cary3 dual-beam spectrophotometer. On the other hand, thrombin present at the same concentration generates an absorbance signal of released p-nitroaniline after 10 minutes that is indistinguishable from noise. When using S2266, the signal due to substrate cleavage by thrombin far exceeds that due to cleavage by APC over the same time scale.
Hydrolysis of 60 μmol/L H-D-Trp-Arg-Arg-p-nitroanilide (•, ▪) or S2266 (○, □) by 1 nmol/L human APC (•, ○) or human thrombin (▪, □). Data points depict the absorbance at 405 nm due to the released p-nitroaniline (ε405 = 9,920 mol/L−1cm−1) from the cleaved substrate. Experimental conditions are 5 mmol/L Tris, 0.1% PEG, 200 mmol/L NaCl, pH 8.0, at 25°C. When using H-D-Trp-Arg-Arg-p-nitroanilide, APC at a concentration of 1 nmol/L can easily be detected and gives an absorbance signal of released p-nitroaniline after 10 minutes that is 160-fold the noise level of ±0.0001 OD units (or ±10 nmol/L p-nitroaniline) of a Cary3 dual-beam spectrophotometer. On the other hand, thrombin present at the same concentration generates an absorbance signal of released p-nitroaniline after 10 minutes that is indistinguishable from noise. When using S2266, the signal due to substrate cleavage by thrombin far exceeds that due to cleavage by APC over the same time scale.
ACKNOWLEDGMENT
Supported by National Institutes of Health Research Grant No. HL49413 and a grant from the American Heart Association. E.D.C. is an Established Investigator of the American Heart Association and Genentech.