Abstract
To investigate whether resistance to activated protein C (APC resistance) because of a mutation in the factor V gene (factor V Leiden) leads to a decrease in life expectancy, we analyzed overall and cause-specific mortality in 171 parents whose offspring carried this mutation. Compared with the Dutch general population, and after adjustment for age, sex, and calendar period, we found no excess deaths in the parents (standardized mortality ratio [SMR], 1.0; 95% confidence interval [CI], 0.8 to 1.2). The cause-specific SMR for malignant neoplasms (1.0; 95% CI, 0.6 to 1.4), diseases of the circulatory system (1.0; 95% CI, 0.7 to 1.4), and cerebrovascular disease (1.0; 95% CI, 0.4 to 1.9) also did not differ from unity. The SMRs for diseases of the respiratory system (1.4; 95% CI, 0.6 to 2.6) and for ischemic heart diseases (1.1; 95% CI, 0.7 to 1.7) were slightly increased. Under the age of 45 years, there was a ninefold increase of dying from ischemic heart disease. Thromboembolic complications were mentioned only once (venous embolism or thrombosis) as an underlying (“primary”) cause of death (SMR, 2.3; 95% CI, 0.1 to 13.0) and three times (pulmonary embolisms) as a contributing (“secondary”) cause of death (SMR, 1.5; 95% CI, 0.3 to 4.4). We conclude that there is no major effect of APC resistance on life expectancy. Therefore, long-term anticoagulation in carriers of factor V Leiden, on the basis of the carrier state alone, is not indicated.
A POOR ANTICOAGULANT response to activated protein C (APC resistance) is a common autosomal inherited abnormality, which is associated with a tendency to venous thrombosis.1-3 This APC resistance is associated with a mutation at the APC cleavage site in the factor V gene (factor V Leiden).4,5 The risk of deep-vein thrombosis in unselected carriers of the mutation is increased eightfold.2,6 Concern for premature death has been raised about the effect of APC resistance by a case report of fatal pulmonary embolism in a 32-year-old man with a profound, and possibly homozygous, APC resistance.7
Life expectancy in individuals with the factor V Leiden mutation can be studied during long-term follow-up periods. However, by genetic reasoning, we know that, because of its autosomal dominant mode, at least one parent of each heterozygous index case carried the abnormality and passed it on to his/her offspring and that, in homozygous carriers, both parents were affected. Therefore, a deleterious effect of the mutation on mortality could also be studied in the parents of a proband with the factor V Leiden mutation. We questioned whether an excess mortality in parents of individuals with APC resistance exists and, especially, whether the risk of dying from thromboembolic complications has been increased. The method used in this study has been extensively described in former reports on mortality in hereditary antithrombin deficiency8 and protein C deficiency.9
MATERIALS AND METHODS
Study population.Our investigation was based on the Leiden Thrombophilia Study (LETS). The design of this population-based case-control study on hereditary venous thrombosis has been specified previously.2 A total of 474 consecutive patients younger than 70 years of age, with an objective diagnosis of a first episode of deep venous thrombosis between 1988 and 1993 and without an underlying malignant disorder, were selected from the files of three anticoagulation clinics in the Netherlands. In 92 of the patients, APC resistance was subsequently confirmed by DNA analysis.5 Using municipal registers and state archives (where, since 1809, all births, marriages, and deaths must be reported by Dutch law), we retrieved the dates of birth and death for the 184 parents of all carriers. Because the parents had to live until the birth of the index case to pass on the mutation, the years lived before this birth were ignored. Therefore, the follow-up period extended from the date of birth of their affected offspring to the date of death or to December 31, 1994.
Causes of death.We were able to survey all causes of death of the parents who were deceased in the Netherlands between 1941 and 1994. These were made available by the Netherlands Central Bureau of Statistics (Voorburg, The Netherlands), where one copy of each death certificate is kept. The causes of death are routinely coded by this Bureau using the coding rules from the International Classification of Diseases, fifth through ninth revisions (ICD-5 through ICD-9).10-14 For each death, both the underlying (primary) cause of death and any other contributing (secondary) causes of death, if listed, were made available. For the analysis, the older ICD codes were recoded to conform to the ninth revision. We classified the deceased persons into six groups of underlying causes of death: malignant neoplasms (ICD-9 codes 1400-2099), diseases of the circulatory system (ICD-9 codes 3900-4599), cerebrovascular diseases (ICD-9 codes 4300-4389), ischemic heart diseases (ICD-9 codes 4100-4149), diseases of the respiratory system (ICD-9 codes 4600-5199), and thromboembolic complications. We defined thromboembolic complications as pulmonary embolism (ICD-9 code 4151), (thrombo)phlebitis (ICD-9 codes 4510-4519), venous embolism or thrombosis (ICD-9 codes 4530-4539), venous complications in pregnancy and the puerperium (ICD-9 codes 6710-6719), and obstetrical pulmonary embolism (ICD-9 codes 6730-6739).
On the death certificate, a maximum of three secondary causes of death is recorded. These conditions may be contributing causes, conditions that are part of the causal pathway, or any other conditions mentioned on the certificate.15 16 Because venous thromboembolic complications are frequently coded as a contributing or fatal cause of death, we also studied the occurrence of these complications within the group of secondary causes of death.
Mackenbach et al16 reported that, between categories of primary causes of death, there are major differences in the prevalence at death of other secondary causes. The number of reports on all secondary causes of death should give us an indication as to whether, in our study group, the frequency of secondary causes of death might be compared with that of the general population.
Statistical analysis.The overall and cause-specific mortality of the parents (observed) was compared with that of the Dutch general population adjusted for age, sex, and calendar period (expected). The ratio of observed to expected number of deaths is the standardized mortality ratio (SMR), which can be viewed as a relative risk. The expected mortality was calculated by multiplying the total number of years lived by the study population per sex, age, and calendar period with the cause-specific population mortality rates from the annual reports of the Netherlands Central Bureau of Statistics, using the computer program “Person-Years” (Prof J. Peto, Institute of Cancer Research, London, UK).17 Confidence limits for the SMR are based on a Poisson distribution for the observed number of deaths.18
The calendar periods were assigned to 5-year intervals from 1941 to 1994 and subdivided by sex and into 5-year age classes. To each of these periods, we applied the overall and cause-specific population mortality rates of the mid-interval year, starting from the mid-interval year 1943 (period, 1941 through 1945), 1948 (period, 1946 through 1950) until 1993 (period, 1991 through 1994). Only since the early 1950s has the number of secondary causes of death been mentioned in the annual reports. Besides, since 1993, the secondary causes of death have no longer been integrated in these reports. These facts imply that the calculation of expected mortality for the secondary causes of death started with the mid-interval year 1953 for the period from 1951 through 1955 until 1992 for the last calendar period. Furthermore, only since 1971 has the total of all secondary causes of death been given in the annual reports; therefore, the expected overall secondary causes of death could only be calculated for the period from 1971 through 1994.
All-cause mortality in 171 parents of affected offspring by calendar periods from date of birth of their affected offspring to the end-of-study date.
All-cause mortality in 171 parents of affected offspring by calendar periods from date of birth of their affected offspring to the end-of-study date.
RESULTS
Characteristics of the study population.For 171 parents (86 mothers and 85 fathers), the dates of birth and the dates of death or the end-of-study dates were analyzed. The parents of 4 probandi had never lived in the Netherlands, and 5 parents could not be traced to the end-of-study date. Year of birth ranged from 1873 to 1948, and year of death ranged from 1943 to 1994. The mean age at the end-of-study date was 66 years (range, 46 to 87 years). The mean age of the parents at which their affected offspring was born was 30 years (range, 18 to 46 years) for the mothers and 32 years (range, 19 to 49 years) for the fathers.
From 1941 until 1994, the 171 parents were followed up over 5,810 person-years, in which 86 deaths took place. A total of 50 men died in 2,769 person-years, and 36 women died in 3,041 person-years. The mean age of death was 70 years (range, 40 to 96 years) in men and 71 years (range, 33 to 96 years) in women. Two men died in foreign countries and 2 death certificates (from 1 man and 1 woman) could not be linked; therefore, for the analysis of the causes of death, 82 death certificates were reviewed. To prevent the introduction of selection bias, the person-years of these 4 parents were excluded from the calculation of the cause-specific expected number of deaths.
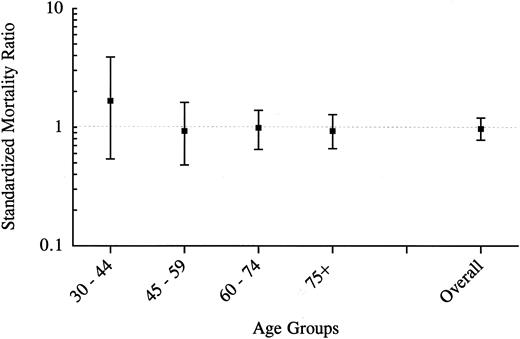
All-cause mortality.The overall mortality in the study group and the general population was equal (SMR, 1.0; 95% confidence interval [CI], 0.8 to 1.2). In men, the SMR was 0.9 (95% CI, 0.7 to 1.2), and in women the SMR was 1.0 (95% CI, 0.7 to 1.4). Figure 1 shows the relative mortality risks for different calendar periods from 1941 to the present. In none of these periods did the SMR significantly differ from unity; although, for the period from 1941 through 1955, 10 deaths were observed, whereas 7.3 deaths had been expected (SMR, 1.4; 95% CI, 0.7 to 2.5). Moreover, in none of the age groups was there a significant difference between the observed and expected mortality (Fig 2). However, 5 parents (2 men and 3 women) died in the youngest age category, including a man who died in World War II and a woman with an unknown cause of death (SMR, 1.7; 95% CI, 0.5 to 3.9).
All-cause mortality in 171 parents of affected offspring by age groups between 1941 and 1994.
All-cause mortality in 171 parents of affected offspring by age groups between 1941 and 1994.
Causes of death.The observed number of deaths from malignant neoplasms, circulatory diseases, and cerebrovascular diseases matched the expected number of deaths. The SMRs for diseases of the respiratory system and for ischemic heart diseases were slightly increased (Table 1). There were 2 deaths from ischemic heart disease before the age of 45 years, whereas only 0.2 deaths were expected before this age (SMR, 9.2; 95% CI, 1.1 to 32.8). Thromboembolic complications were mentioned only once (venous embolism or thrombosis) as a primary cause of death. However, because this cause of death is even more rare in the general population, this number was much higher than expected, although with a very broad confidence interval (SMR, 2.3; 95% CI, 0.1 to 13; see Table 1).
The number of reports on all secondary causes of death was about equal to that of the general population, indicating that the cause-specific secondary mortality rates were informative. Among these contributing causes of death, 3 were classified as pulmonary embolism (Table 2), including the 1 caused by the venous thromboembolism; 2 of them were men in the age group of 45 through 59 years, and 1 was a woman in the age group of 60 through 74 years.
DISCUSSION
We studied the overall mortality in parents whose children were carriers of the factor V Leiden mutation. No excess mortality was found, and confidence limits were narrow. Obviously, only half of the parents of heterozygous and both parents of homozygous patients will have carried the mutation at the APC cleavage site in the factor V gene. Among the 171 individuals, 14 were the parent of 7 homozygous patients. Therefore, at least 92 of 171 individuals studied were themselves carriers of the defect. The inclusion of a substantial number of “normal” parents might have diluted any effect on mortality but cannot explain the absence of any increased mortality risk. This is so because the diluted SMR is composed of half of the SMR of the APC resistant individuals plus half of the SMR of normal persons. Because our observed SMR was 1 and, by definition, the SMR of normal persons is 1, the SMR of the APC-resistant individuals can also only be 1. However, had we found an SMR of 3, this diluted observed SMR would then point to a true SMR of 5 for the APC-resistant individuals.
From the three major categories of specific causes of death (malignant neoplasms and diseases of the circulatory and of the respiratory systems), only the number of deaths from respiratory diseases was slightly increased. We can roughly split these diseases into chronic obstructive pulmonary diseases and pneumonia. It is possible that reports of mortality by pneumonia could be caused by missed pulmonary embolism. However, among these 9 observed deaths caused by respiratory diseases, only 1 was an “unspecified pneumonia.”
Ischemic heart disease gave excess mortality under 45 years of age. Although based on only 2 deaths, it might point to the factor V Leiden mutation as a risk factor for arterial thrombosis in relatively young individuals. This finding is supported by the findings of some other investigators but possibly is only pronounced in homozygous individuals.19-21 However, there are just as many studies that do not find an association.22-25
If small, an effect on the risk of death of thromboembolic complications may not be apparent from the overall figures. Among 86 deaths, only 1 was classified as being caused by thromboembolism. Even though this 1 event exceeded the expected number for this cohort (because of the rareness of fatal thromboembolisms in the general population), this does not point to a high rate of deaths by thrombotic causes. Similarly, the 3 deaths in which pulmonary embolism was listed as a secondary cause of death are not sufficient enough to cause alarm.
Given our sample size, if we had found 3 or more primary deaths from thromboembolic complications, it would have given us an SMR significantly greater than unity. Such a rate would have meant a clear deleterious effect of the mutation on life expectancy. At present, an increase in mortality, if present at all, can only be very small. To quantify more precisely such a small increase in mortality from thromboembolic complications caused by the factor V Leiden mutation, a study about 10 times larger would have been necessary.
In theory, these fatal thromboembolisms might have been prevented by long-term prophylactic anticoagulation treatment. However, it is not likely that this would have improved the overall survival rates. On the contrary, previous studies estimated the incidence of major bleeding episodes during oral anticoagulant therapy to be 2% to 3% per year.26,27 Furthermore, the risk of fatal hemorrhage brought about by anticoagulant therapy was reported to be 6 per 1,000 treatment-years.27 Because our total follow-up time was nearly 6,000 person-years, long-term prophylactic anticoagulation treatment would have induced a considerable number of fatal hemorrhages in our cohort, leading to excess mortality. Even for the use of prophylaxis restricted to high-risk situations, such as pregnancy or the puerperium, it is not obvious whether the induced complications outweigh the thrombotic risk in individuals who have never experienced a thrombosis.28 Because we have no information about the parents' medical histories, it can not be eliminated that some of them were treated with anticoagulants for shorter or longer periods for recurrent thrombosis. The time frame of the follow-up study renders it unlikely that this included a substantial number of individuals. The absence of excess mortality by hemorrhage in our study also does not point to widespread use of anticoagulants.
Our findings are in accordance with our previous reports on thrombophilia caused by hereditary antithrombin8 and protein C deficiency,9 in which we also did not find any indication of excess deaths related to the abnormality. However, in contrast to thrombophilia associated with the deficiency of protein C, protein S, or antithrombin, APC resistance is common. The carrier rate in Europe is estimated at 2% to 7%2,3,5,29 and may be the result of evolutionary advantages for the heterozygous state at the time that thrombophilia may have been less damaging because of the absence of some lifestyle risk factors. A study by Mari et al30 among healthy centenarians supported our findings; they found similar allele frequencies in centenarians and noncentenarians, suggesting that the factor V Leiden mutation is compatible with extreme longevity.
Because of the high prevalence of factor V Leiden, it may be feasible to confirm our results in a prospective follow-up study and to quantify the association between the factor V Leiden mutation and arterial and venous thromboembolism with and without fatal outcome. Our results apply only to heterozygous factor V Leiden carriership, and it remains to be elucidated whether the homozygous state confers an additional risk, as it does for nonfatal venous thrombosis (80-fold).31 It may well be worthwhile to test the families of carriers of the factor V Leiden mutation for the defect for clinical management of thrombosis, but there is no need for major concern about a deleterious effect on life expectancy.
ACKNOWLEDGMENT
We thank Dr T. Koster (Department of Internal Medicine, University Hospital Leiden, Leiden, The Netherlands) for obtaining the data of the APC-resistant patients in the Leiden Thrombophilia Study (LETS). We thank the Department of Health Statistics of the Netherlands Central Bureau of Statistics for generously making available the mortality statistics and database linking.
Supported by a grant from the Netherlands Organization for Scientific Research (NWO Grant nr 900-561-063).
Address reprint requests to Frits R. Rosendaal, MD, PhD, Department of Clinical Epidemiology, University Hospital Leiden, Building 1 CO-P, P.O. Box 9600, 2300 RC Leiden, The Netherlands.