Abstract
The susceptibility of recombinant type 2A von Willebrand factor (vWF ) to a recently identified plasma metalloproteinase and the potential application of proteolysis inhibition in the treatment of the disease were investigated. Two recombinant type 2A vWF mutants, R834W and R834Q, were spontaneously cleaved by the partially purified plasma proteinase to smaller forms. When treated with guanidine HCl, both the wild-type and the R834W mutant vWF exhibited a biphasic change in proteolytic susceptibility, reaching the same maximum cleavage at 1.25 mol/L guanidine HCl. Proteolysis of the recombinant vWF generated the same 350-kD and 200-kD species (dimers of the 176-kD and 140-kD fragments, respectively) as those found in normal plasma. The proteinase activity was inhibited by doxycycline, with an IC50 of approximately 0.25 mmol/L. The inhibitory activity of doxycycline was related to its metallic cation binding activity. Susceptibility of the recombinant vWF to the proteinase was inhibited by monoclonal antibody VP-1 (directed against residues 828-842 of the vWF polypeptide), but not by two other monoclonal antibodies M13 and M31. The spontaneous susceptibility to proteolytic cleavage may account for the lack of large multimers in type 2A von Willebrand disease (vWD), and the results with tetracyclines and monoclonal antibody VP-1 offer new strategies for developing specific treatment of type 2A vWD.
THE SIZE OF VON WILLEBRAND factor (vWF ) is a major determinant of its activity in supporting platelet adhesion and aggregation at sites of vessel injury. Results from recent studies have suggested a model of how the sizes of plasma vWF multimers are regulated. Although vWF is released from endothelial cells as an extra large polymer,1-3 it is cleaved at the Y842/M843 peptide bond4 and other minor sites by a plasma proteinase into the plasma series of multimers.5,6 Cleavage at the Y842/M843 peptide bond leads to the generation of homodimers of a 140-kD and a 176-kD fragment whose presence in normal plasma has been identified in several laboratories.7-9 The interaction of vWF with the proteinase is dependent on the conformation of vWF molecules.5,6 Thus, whereas cleavage of vWF does not occur appreciably when normal plasma is incubated in a test tube, a markedly increased cleavage occurs when purified vWF is exposed to high shear stress5 or guanidine HCl6 before incubation with the proteinase. The plasma proteinase that cleaves vWF to generate dimers of the 176-kD and the 140-kD fragments has been partially purified and characterized.6,10 The proteinase, with a molecular weight of approximately 200 kD, is distinct from the matrix metalloproteinase family11 in that it is active in the plasma5 and is not affected by batimastat,6 a potent inhibitor of matrix metalloproteinases.12
Type 2A von Willebrand disease (vWD) is characterized by a decrease of the large multimers in the plasma. Many mutations associated with type 2A vWD cluster within the A2 homologous repeat,13 where the T842/M843 bond is located. Expression of mutant vWF sequences by transfection in mammalian cells has led to the classification of type 2A vWD into two distinct subgroups.13 In group 1, the point mutation leads to a defect in intracellular transport. In contrast, group 2 vWF released in tissue cultures is not different from wild-type (WT) vWF in size distribution.
In some type 2A patients, the vWF is presumed to be susceptible to an EDTA-sensitive proteinase because the large and intermediate multimers are increased when the plasma is collected in EDTA with or without leupeptin and N-ethylmaleimide.14,15 The plasma vWF of patients with type 2A vWD contains the same 176-kD and 140-kD proteolytic fragments as those in the normal plasma, although they are increased relative to the intact 225-kD subunit. These observations lead to the hypothesis that the decrease of large multimers in type 2A patients is caused by excessive proteolysis. However, because the smaller multimers of plasma vWF contain a higher fraction of the proteolytic fragments than the larger multimers, an apparent increase in proteolytic fragments can also result from a selective loss of large multimers by nonproteolytic mechanisms.8 Selective binding of large multimers is believed to occur spontaneously in vivo in the type 2B vWD or platelet-type pseudo-vWD and may also occur under high shear stress conditions.16
In this study, we present direct evidence to support that loss of large multimers in type 2A vWF is due to excessive proteolytic susceptibility rather than selective adsorption. Because recombinant type 2A group 2 vWF exhibits normal activity in supporting platelet adhesion,17 measures that prevent the loss of the large multimers have the potential of correcting the bleeding diathesis. We further explored the inhibitory activity of tetracyclines on the proteinase as well as the protective effect of VP-1, a monoclonal anti-vWF antibody, against the proteolytic cleavage.
MATERIALS AND METHODS
Reagents and materials.Monoclonal antibodies (MoAbs) M13, VP-1, and M31, directed respectively against amino acid residues 631-710, 828-842, and 1481-1693 of the vWF polypeptide,18 19 were a generous gift from Dr Z.M. Ruggeri (Scripps Research Institute, La Jolla, CA). Each antibody, as determined by enzyme-linked immunosorbent assay, contained approximately 90 μmol/L, 93 μmol/L, and 306 μmol/L mouse IgG, respectively. A rabbit IgG polyclonal antibody against human vWF was purchased from Dako Corp (Carpinteria, CA). Lyophilized platelets and ristocetin were purchased from Bio/Data Corp (Horsham, PA). Electrophoresis reagents were purchased from Bio-Rad (Richmond, CA). Peptide SP-2, consisting of the amino acid residues 828-842 of the vWF polypeptide (NH2 -LVSQGDREQAPNLVY-COOH), was synthesized by Bio-Synthesis Inc (Lewisville, TX). The peptide, with a purity of 94% after high-performance liquid chromatography on a Poros R2M column, was dissolved in distilled water and stored in aliquots at −70°C. All other reagents were from Sigma Chemical Co (St Louis, MO).
Recombinant vWF.WT vWF (0.7 to 1.0 μg/mL) and R834W mutant vWF (0.2 to 0.4 μg/mL) secreted in the culture media of transiently transfected COS-7 cells were used in these studies.20 The culture media were collected into a final concentration of 5 mmol/L EDTA and 2 mmol/L phenylmethylsulfonyl fluoride. Before incubation with the proteinase, the media were recalcified to a final concentration of 10 mmol/L CaCl2 . WT vWF (36 μg/mL) and R834W (34 μg/mL) and R834Q (29 μg/mL) vWF mutants were also expressed in Baby Hamster Kidney (BHK) cells that were stably cotransfected with furin cDNA and purified by affinity chromatography as previously described.17 The vWF was suspended in Tris-buffered saline (TBS; 50 mmol/L Tris HCl, 100 mmol/L NaCl, pH 7.4) and saved at −70°C.
Ristocetin cofactor activity was determined in a platelet aggregometer by using reconstituted lyophilized normal human platelets at a final concentration of 200 × 103/μL and 1.2 mg/mL ristocetin.
vWF proteinase.The vWF proteinase in 50 mmol/L Tris HCl, 50 mmol/L NaCl, pH 8.0 (specific activity = 5 U/mg protein; 1 U = the activity in 1 mL heparinized normal plasma cryosupernatant) was partially purified approximately 900-fold from heparinized normal plasma by gel filtration on Sephacryl S300 HR, Matrex gel orange A dye affinity chromatography, and anion exchange chromatography on Q-Sepharose, as previously described.6 In some experiments, proteinase fractions eluted from orange A agarose column and dialyzed against TBS were used. The determination of proteinase activity is described below.
Proteolytic cleavage of vWF.Recombinant vWF in COS-7 cell culture media was incubated at 1:1 (vol/vol) with the proteinase (0.4 U/mL) or its serial dilutions, in the presence or absence of recalcification, at 37°C for 60 minutes. Affinity-purified BHK vWF, diluted 1:1 (vol/vol) with TBS containing designated concentrations of guanidine HCl, was incubated at 1:9 (vol/vol) with the proteinase (0.5 to 1.0 U/mL) at 37°C. The reactions were stopped by adding 10 mmol/L EDTA and 2 vol (for the cell culture media) or 4 vol (for the purified vWF ) of gel sample buffer.
To determine the inhibitory activity of tetracyclines (each was prepared on the day of experiment as a 100 mmol/L solution or its dilutions in distilled water or, for minocycline, as a 10 mmol/L solution in 2% dimethyl sulfoxide/distilled water), the proteinase was incubated with 1/9 vol of tetracyclines at designated concentrations for 30 minutes at room temperature before vWF was added. Plasmin at 0.015 to 0.5 U/mL (1 U = ΔA275 of 1.0 from α-casein in 20 minutes at pH 7.5, 37°C) in 25 mmol/L Tris Hcl, 0.15 mol/L NaCl, pH 7.5, was treated with tetracyclines similarly. In some experiments, to exclude the possibility that inhibition of proteolysis resulted from interaction of doxycycline with the vWF rather than the proteinase, proteinase/doxycycline mixtures, as well as control proteinase/distilled water, were dialyzed against two changes of 1,000× volumes of Tris saline, pH 8.0, for 24 hours before the addition of vWF. To investigate whether the inhibitory activity of doxycycline was related to its binding avidity for metallic cations, the antibiotic was treated with ZnCl2 , CuSo4 , or CaCl2 at room temperature for 15 minutes before incubation with the proteinase. The proteinase/doxycycline mixtures were then made to contain the same final concentration of CaCl2 , ZnCl2 , or CuSO4 before the vWF was added.
To study the effect of MoAb VP-1 on vWF cleavage by the plasma proteinase, the vWF was incubated with 1/9 vol of appropriately diluted VP-1 at room temperature for 15 minutes before the proteinase was added. MoAbs M13 and M31 were similarly tested as controls. The specificity of VP-1 inhibitory activity was further investigated by treating the antibody with designated concentrations of the synthetic peptide SP-2 for 30 minutes at room temperature before it was added to the vWF.
Gel analysis of vWF.The multimer size distribution was analyzed by electrophoresis in 1.5% agarose gels or in 1.6% low gelling temperature agarose gels with discontinuous buffers. Proteolytic fragments resulting from the proteolysis were analyzed by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under either nonreducing or reducing conditions. Prestained molecular weight standards of high range (Bio-Rad), fibrinogen (molecular weight [MW] 340,000), plasma fibronectin (MW 520,000), and IgM (MW 970,000) were used as reference for MW determination. Normal plasma vWF was run simultaneously to help identification of the proteolytic fragments generated from the recombinant vWF. These techniques, as well as the densitometric analysis of the autoradiographs, were performed as previously described.5 Briefly, for 1.5% SDS agarose gel electrophoresis in a 50 mmoL/L sodium phosphate buffer system, vWF samples were incubated at 37°C for 120 minutes at designated dilutions with a sample buffer containing 10 mmol/L sodium phosphate, pH 7.0, and a final concentration of 2 gm% SDS before loading onto the wells of a 24 × 10 cm (width × length) gel. Electrophoresis was performed under 100 V constant voltage in a horizontal electrophoresis cell (Bio-Phoresis; Bio-Rad) at 15°C, using 100 mmol/L sodium phosphate, pH 7.0, and 0.1% SDS as the electrode buffer. At the end of electrophoresis, the gel was incubated for 60 minutes in 25% isopropanol/10% acetic acid and washed extensively in distilled water for 2 hours before it was allowed to react overnight in TBS containing 2 mg/mL bovine γ-globulin with 1 μCi of an affinity-purified 125I-labeled polyclonal rabbit antihuman vWF antibody. The gel was then washed with two changes of TBS and one change of distilled water over 48 hours before it was dried for autoradiography. Normalized peak distance of each lane on SDS agarose gel was determined from the distance between the sample starting point and the peak on the densitometric tracing, divided by that of the control vWF incubated with TBS. In tracings with a plateau instead of a peak, the middle point of the plateau was used as the peak. Normalized peak distance was used as a measure of the multimer size distribution. The proteinase activity of a sample was determined by interpolating the 200-kD fragment band intensity in the vWF/sample mixture against those in the mixtures of the same vWF and serial dilutions of heparinized normal plasma cryosupernatant (1/2, 1/3, and 1/4). Curve-fitting using natural cubic spline was performed on a computer software PSI-PLOT (Poly Software International, Salt Lake City, UT). Difference between groups of data were determined by the Student's t-test.
RESULTS
The multimer size distribution of recombinant WT vWF and R834W mutant expressed in COS-7 cells is shown in Fig 1A, lanes 1 and 2. Both vWF have the same multimer composition, consisting of prominent extra large forms and some small forms. The large forms of both the WT (lanes 3 through 5) and the R834W mutant (lanes 9 through 11) were converted into smaller forms after incubation with the proteinase, with the vWF mutant exhibiting more size reduction. The large forms of both the WT and the R834W mutant remained unchanged when incubation with the proteinase was performed in the presence of EDTA (lanes 6 through 8 and lanes 12 through 14).
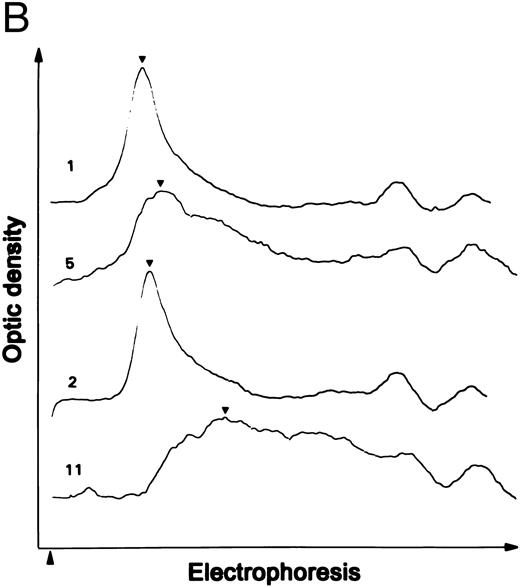
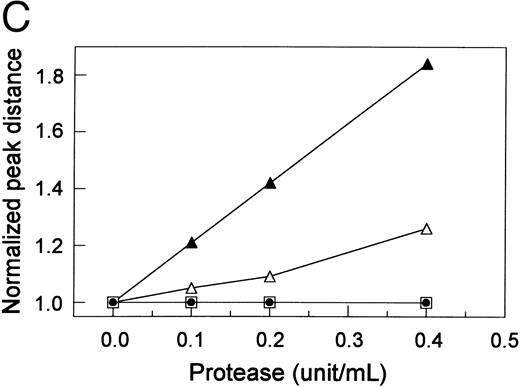
Proteolytic cleavage of recombinant vWF expressed in COS-7 cells. (A) Autoradiograph of a 1.5% SDS agarose gel probed with 125I-labeled anti-vWF. Lane N, vWF in normal plasma. Lanes 1 and 2, recombinant WT vWF and R834W vWF mutant in the culture media of COS-7 cells. Lanes 3 through 5 and lanes 9 through 11, WT vWF (lanes 3 through 5) and R834W vWF mutant (lanes 9 through 11) after 60 minutes of incubation at 37°C with the plasma proteinase at 0.1 U/mL (lanes 3 and 9), 0.2 U/mL (lanes 4 and 10), and 0.4 U/mL (lanes 5 and 11). Lanes 6 through 8 and lanes 12 through 14, same as lanes 3 through 5 and lanes 9 through 11, respectively, except that the incubation was performed without prior recalcification of the vWF samples. (B) Representative densitometric tracings of lanes 1, 2, 5, and 11 in (A). (▴) The sample starting point; (▾) the peaks of the tracings. (C) The normalized peak distances of the lanes shown in (A) for recalcified WT vWF (▵) or R834W mutant (▴) and nonrecalcified WT vWF (□) or R834W mutant (•) were plotted against the proteinase concentration that the vWF sample was exposed to.
Proteolytic cleavage of recombinant vWF expressed in COS-7 cells. (A) Autoradiograph of a 1.5% SDS agarose gel probed with 125I-labeled anti-vWF. Lane N, vWF in normal plasma. Lanes 1 and 2, recombinant WT vWF and R834W vWF mutant in the culture media of COS-7 cells. Lanes 3 through 5 and lanes 9 through 11, WT vWF (lanes 3 through 5) and R834W vWF mutant (lanes 9 through 11) after 60 minutes of incubation at 37°C with the plasma proteinase at 0.1 U/mL (lanes 3 and 9), 0.2 U/mL (lanes 4 and 10), and 0.4 U/mL (lanes 5 and 11). Lanes 6 through 8 and lanes 12 through 14, same as lanes 3 through 5 and lanes 9 through 11, respectively, except that the incubation was performed without prior recalcification of the vWF samples. (B) Representative densitometric tracings of lanes 1, 2, 5, and 11 in (A). (▴) The sample starting point; (▾) the peaks of the tracings. (C) The normalized peak distances of the lanes shown in (A) for recalcified WT vWF (▵) or R834W mutant (▴) and nonrecalcified WT vWF (□) or R834W mutant (•) were plotted against the proteinase concentration that the vWF sample was exposed to.
The shift in size distribution was further investigated by scanning densitometry. Representative densitometric tracings of lanes 1, 2, 5, and 11 are shown in Fig 1B. The normalized peak distance was plotted against the proteinase concentration (Fig 1C). The proteinase produced a longer peak distance or more size reduction in the R834W mutant than the WT vWF. The mutant vWF was approximately four times more sensitive to the proteinase than the WT vWF, because its peak distance after treatment with 0.1 U/mL proteinase was similar to that of the WT vWF treated with 0.4 U/mL proteinase.
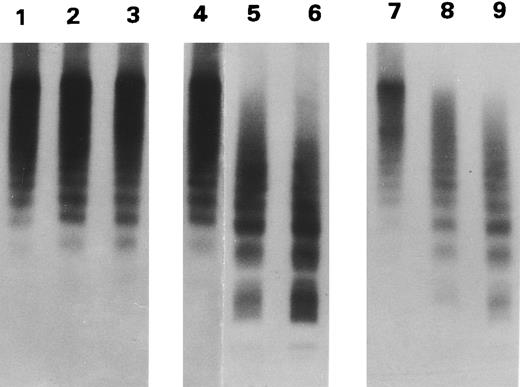
The BHK vWF did not contain small forms (Fig 2). Because the BHK cells were cotransfected with furin, the vWF did not contain pro-vWF (not shown). Again, there was no difference between the WT and the two mutant vWF in the size distribution (Fig 2, lanes 1, 4, and 7). After incubation with the proteinase, the WT vWF was only minimally cleaved (lanes 2 and 3). In contrast, the large forms of both R834W (lanes 5 and 6) and R834Q (lanes 8 and 9) mutants were replaced by intermediate and small forms.
Proteolytic cleavage of recombinant vWF expressed in BHK cells. Autoradiograph of a 1.5% SDS agarose gel probed with 125I-labeled anti-vWF. Recombinant WT vWF (lanes 1 through 3), R834W vWF mutant (lanes 4 through 6), and R834Q vWF mutant (lanes 7 through 9), purified from the culture media of transfected BHK cells, were incubated with either TBS (lanes 1, 4, and 7) or with 1 U/mL of the plasma proteinase for 15 minutes (lanes 2, 5, and 8) and 30 minutes (lanes 3, 6, and 9).
Proteolytic cleavage of recombinant vWF expressed in BHK cells. Autoradiograph of a 1.5% SDS agarose gel probed with 125I-labeled anti-vWF. Recombinant WT vWF (lanes 1 through 3), R834W vWF mutant (lanes 4 through 6), and R834Q vWF mutant (lanes 7 through 9), purified from the culture media of transfected BHK cells, were incubated with either TBS (lanes 1, 4, and 7) or with 1 U/mL of the plasma proteinase for 15 minutes (lanes 2, 5, and 8) and 30 minutes (lanes 3, 6, and 9).
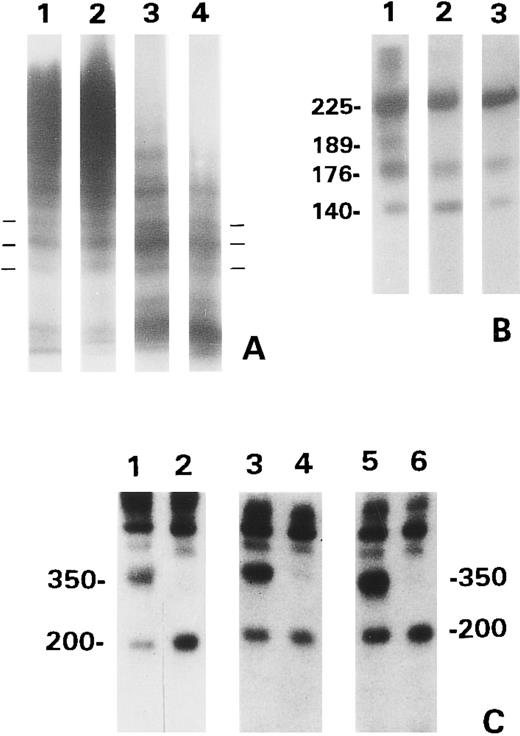
To generate sufficient proteolytic fragments for analysis, the WT vWF was pretreated with guanidine HCl before digestion with the proteinase. When analyzed by SDS low gelling temperature agarose gel electrophoresis with discontinuous buffers, the proteolytic products of each recombinant vWF exhibited the same triplet composition as normal plasma vWF (Fig 3A). In each of the mutants, the concentration of the multimers increased as the size decreased. This resulted in an apparent difference in the banding patterns at the gel front. However, upon prolonged autoradiography, both plasma or WT vWF exhibited the same bands as the vWF mutants. The proteolytic products of the vWF mutants, when analyzed by SDS-PAGE under reducing conditions and immunoblotting with polyclonal anti-vWF, contained the same 176-kD and 140-kD fragments as that of the WT vWF treated with 1.5 mol/L guanidine HCl (Fig 3B). The 189-kD fragment was generated from the WT vWF/guanidine HCl, but not from either of the two mutants. SDS-PAGE under nonreducing conditions and immunoblotting (Fig 3C) showed that the same 350-kD and 200-kD fragments as those found in normal plasma were produced from each recombinant vWF. The 200-kD species, but not the 350-kD species, from each recombinant vWF was recognized by MoAb VP-1 (lanes 2, 4, and 6). No proteolytic fragments were detected in the vWF incubated with TBS (not shown).
Gel analysis of the proteolytic products generated from the BHK recombinant vWF. (A) Normal plasma vWF (lane 1) and the proteolytic products of WT vWF treated with guanidine HCl (lane 2) and R834W (lane 3) and R834Q mutants (lane 4) analyzed in SDS 1.6% low gelling temperature agarose gel. The horizontal bars marked one of triplet multimers. (B) Proteolytic fragments of WT vWF/guanidine HCl (lane 1) and R834W (lane 2) and R834Q mutants, analyzed by 6% SDS-PAGE under reducing conditions. The blot was probed with anti-vWF and 125I-labeled goat antirabbit IgG. The numbers indicate the MW (×10−3). (C) Proteolytic fragments of WT vWF/guanidine HCl (lanes 1 and 2), R834W mutant (lanes 3 and 4), and R834Q mutant (lanes 5 and 6), analyzed by 6% SDS-PAGE under nonreducing conditions. The vWF products were either analyzed directly (lanes 1, 3, and 5) or subjected to immunoisolation with monoclonal VP-1 before electrophoresis (lanes 2, 4, and 6). The blots were probed as in (B).
Gel analysis of the proteolytic products generated from the BHK recombinant vWF. (A) Normal plasma vWF (lane 1) and the proteolytic products of WT vWF treated with guanidine HCl (lane 2) and R834W (lane 3) and R834Q mutants (lane 4) analyzed in SDS 1.6% low gelling temperature agarose gel. The horizontal bars marked one of triplet multimers. (B) Proteolytic fragments of WT vWF/guanidine HCl (lane 1) and R834W (lane 2) and R834Q mutants, analyzed by 6% SDS-PAGE under reducing conditions. The blot was probed with anti-vWF and 125I-labeled goat antirabbit IgG. The numbers indicate the MW (×10−3). (C) Proteolytic fragments of WT vWF/guanidine HCl (lanes 1 and 2), R834W mutant (lanes 3 and 4), and R834Q mutant (lanes 5 and 6), analyzed by 6% SDS-PAGE under nonreducing conditions. The vWF products were either analyzed directly (lanes 1, 3, and 5) or subjected to immunoisolation with monoclonal VP-1 before electrophoresis (lanes 2, 4, and 6). The blots were probed as in (B).
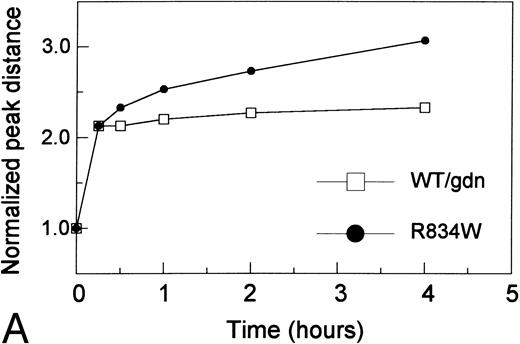
To further explore the difference between vWF mutants and WT vWF treated with guanidine HCl, the kinetics of proteolysis, as measured by the shift of peak distance, of the R834W mutant was compared with that of the WT vWF treated with 1.25 mol/L guanidine HCl (Fig 4A). Similar and swift cleavage occurred in the first 15 to 30 minutes. Upon further incubation, cleavage of the mutant vWF continued, whereas that of guanidine HCl-treated WT vWF proceeded only slightly.
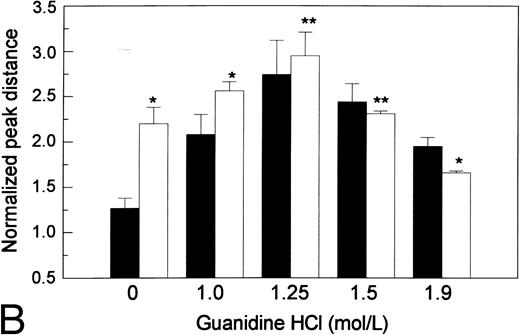
The effects of guanidine HCl on proteolytic susceptibility of the recombinant vWF. (A) Difference in size reduction kinetics, represented by increased normalized peak distance on densitometric tracings of an SDS 1.5% agarose gel, of purified recombinant WT vWF in 1.25 mol/L guanidine HCl (WT/gdn, □) or R834W vWF mutant in TBS (R834W, •) incubated with 1 U/mL proteinase. The reaction was stopped at designated intervals by 10 mmol/L EDTA and SDS agarose gel electrophoresis was performed. (B) Guanidine HCl-dependent size reduction, represented by increased normalized peak distance on densitometric tracings of SDS 1.5% agarose gels. Purified recombinant WT vWF (▪) and R834W vWF mutant (□) were treated with designated concentrations of guanidine HCl before incubation with the plasma proteinase at 1/10 dilution. The reaction was stopped at 30 minutes by 10 mmol/L EDTA. *P < .05, **P < .2.
The effects of guanidine HCl on proteolytic susceptibility of the recombinant vWF. (A) Difference in size reduction kinetics, represented by increased normalized peak distance on densitometric tracings of an SDS 1.5% agarose gel, of purified recombinant WT vWF in 1.25 mol/L guanidine HCl (WT/gdn, □) or R834W vWF mutant in TBS (R834W, •) incubated with 1 U/mL proteinase. The reaction was stopped at designated intervals by 10 mmol/L EDTA and SDS agarose gel electrophoresis was performed. (B) Guanidine HCl-dependent size reduction, represented by increased normalized peak distance on densitometric tracings of SDS 1.5% agarose gels. Purified recombinant WT vWF (▪) and R834W vWF mutant (□) were treated with designated concentrations of guanidine HCl before incubation with the plasma proteinase at 1/10 dilution. The reaction was stopped at 30 minutes by 10 mmol/L EDTA. *P < .05, **P < .2.
The response of the proteolytic susceptibility to guanidine HCl was investigated by treating the vWF with guanidine HCl at the designated concentration before it was incubated with the proteinase (Fig 4B). The means ± SD of normalized peak distances from three separate experiments were depicted. Although the R834W mutant was originally more susceptible to the proteinase than the WT, the latter was more responsive than the mutant to guanidine HCl. As a result, WT vWF and the R834W mutant reached the same maximum proteolytic susceptibility at the same 1.25 mol/L guanidine HCl concentration. As guanidine HCl concentration further increased, cleavage of both WT and mutant vWF decreased. At 1.9 mol/L guanidine HCl, the mutant vWF became slightly less susceptible than the WT vWF.
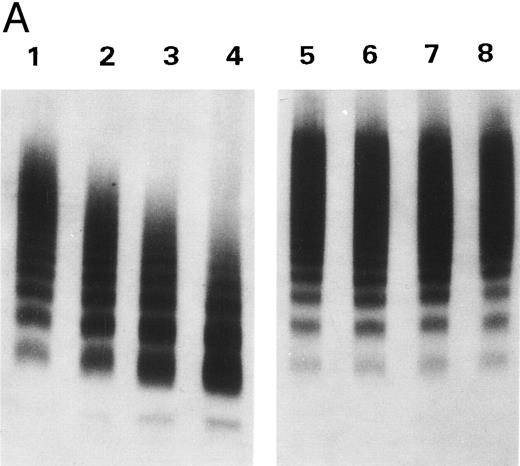
To study the inhibitory effect of doxycycline on the proteinase activity, the proteinase was treated with 2 mmol/L doxycycline before R834W vWF was added. As shown in Fig 5A, cleavage of the vWF mutant was markedly inhibited by doxycycline. The inhibition by doxycycline was not due to its interaction with vWF, because dialysis of the proteinase/doxycycline mixtures before addition of vWF did not abolish the inhibition. Doxycycline up to 2 mmol/L exhibited no effect on the cleavage of vWF by plasmin (data not shown).
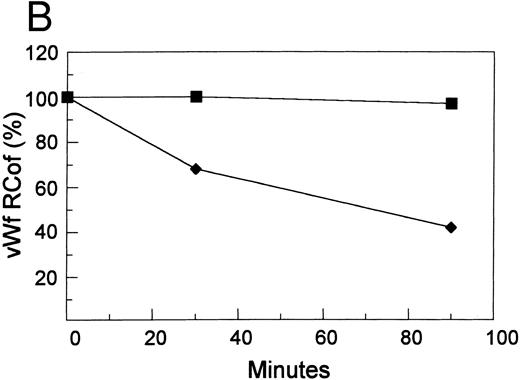
Inhibition by doxycycline of vWF proteolysis. (A) Doxycycline-inhibited proteolytic cleavage of R834W vWF mutant. Autoradiographs of a 1.5% SDS agarose gels probed with 125I-labeled anti-vWF. Lanes 1 through 4 and lanes 5 through 8, R834W vWF mutant incubated with 1 U/mL proteinase without and with 2 mmol/L doxycycline, respectively, for 15 minutes (lanes 1 and 5), 30 minutes (lanes 2 and 6), 60 minutes (lanes 3 and 7), and 90 minutes (lanes 4 and 8). (B) Doxycycline prevented the decrease of R834W vWF Rcof activity by the plasma proteinase. vWF ristocetin cofactor (RCof) was expressed as the percentage of the vWF incubated with TBS. (▪) R834W vWF + proteinase treated with 2 mmol/L doxycycline. (♦) R834W vWF + proteinase.
Inhibition by doxycycline of vWF proteolysis. (A) Doxycycline-inhibited proteolytic cleavage of R834W vWF mutant. Autoradiographs of a 1.5% SDS agarose gels probed with 125I-labeled anti-vWF. Lanes 1 through 4 and lanes 5 through 8, R834W vWF mutant incubated with 1 U/mL proteinase without and with 2 mmol/L doxycycline, respectively, for 15 minutes (lanes 1 and 5), 30 minutes (lanes 2 and 6), 60 minutes (lanes 3 and 7), and 90 minutes (lanes 4 and 8). (B) Doxycycline prevented the decrease of R834W vWF Rcof activity by the plasma proteinase. vWF ristocetin cofactor (RCof) was expressed as the percentage of the vWF incubated with TBS. (▪) R834W vWF + proteinase treated with 2 mmol/L doxycycline. (♦) R834W vWF + proteinase.
The ristocetin cofactor activity before and 30 and 90 minutes after incubation with the proteinase was determined and shown in Fig 5B. Incubation of the R834W mutant was accompanied by a progressive decline in its ristocetin cofactor activity. In contrast, the ristocetin cofactor activity of the vWF incubated with the proteinase treated with 2 mmol/L doxycycline remained essentially unchanged.
The residual activity of the proteinase treated with various concentrations of doxycycline was plotted against the concentrations of doxycycline. With several partially purified proteinase preparations, the IC50 was approximately 0.25 to 0.5 mmol/L. It was 0.5 to 1.0 mmol/L in heparinized plasma cryosupernatant. With the same partially purified proteinases, the IC50 of tetracycline, oxytetracycline, and minocycline were between 0.5 and 1 mmol/L (data not shown).
Tetracyclines possess binding activity for Zn2+ and other metallic cations.21 To determine whether inhibitory activity of doxycycline was related to its metallic cation binding property, doxycycline was treated with Zn2+, Cu2+, or Ca2+ (Fig 6A). Zn2+ or Cu2+ decreased the inhibitory activity of doxycycline. Higher concentrations of the cations were not tested because they became progressively inhibitory to the proteinase activity. Ca2+ up to equimolar concentrations only minimally blocked the inhibitory activity of doxycycline.
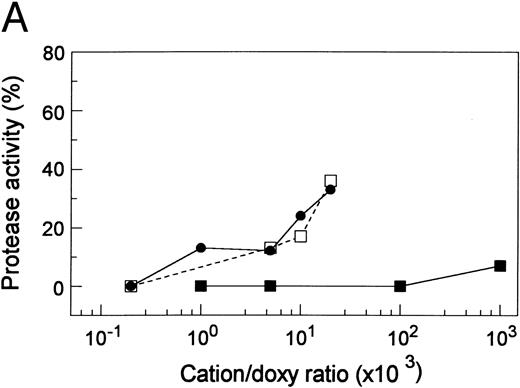
The role of metallic cations in the inhibitory activity of doxycycline. (A) Zn2+ or Cu2+ decreased doxycycline inhibitory activity. Doxycycline (5 mmol/L) was treated with ZnCl2 (•), CuSO4 (□), or CaCl2 (▪) at designated molar concentration ratios before it was added at 1/10 (vol/vol) dilution to the proteinase. Proteinase Inhibition (%) = (Original Activity − Residual Activity)/(Original Activity − Residual Activity After Incubation With Doxycycline Not Treated With Cations) × 100. (B) Restoration of proteinase activity by metallic cations. The plasma proteinase was treated with 1 mmol/L EDTA (♦) or 1 mmol/L doxycycline (□). CaCl2 or ZnCl2 was then added at the designated concentrations. The proteinase activity was plotted against the cation concentrations.
The role of metallic cations in the inhibitory activity of doxycycline. (A) Zn2+ or Cu2+ decreased doxycycline inhibitory activity. Doxycycline (5 mmol/L) was treated with ZnCl2 (•), CuSO4 (□), or CaCl2 (▪) at designated molar concentration ratios before it was added at 1/10 (vol/vol) dilution to the proteinase. Proteinase Inhibition (%) = (Original Activity − Residual Activity)/(Original Activity − Residual Activity After Incubation With Doxycycline Not Treated With Cations) × 100. (B) Restoration of proteinase activity by metallic cations. The plasma proteinase was treated with 1 mmol/L EDTA (♦) or 1 mmol/L doxycycline (□). CaCl2 or ZnCl2 was then added at the designated concentrations. The proteinase activity was plotted against the cation concentrations.
To determine whether the action of doxycycline was different to that of EDTA, the activity of the proteinase treated with 1 mmol/L doxycycline was titrated with Ca2+ or Zn2+ and compared with that treated with 1 mmol/L EDTA and titrated similarly (Fig 6B). Although 3 mmol/L Ca2+ or 2 mmol/L Zn2+ completely reversed the inhibition by EDTA, neither had any effect on the inhibition by doxycycline. Inhibitory activity of Zn2+ per se caused the decline of proteinase activity at Zn2+ greater than 2 mmol/L. In separate but similar experiments, CuSO4 or MgCl2 at the same concentrations did not reverse the inhibition caused by either EDTA or doxycycline (data not shown).
Because MoAb VP-1 is directed against the 15 amino acid residues preceding the cleavage site, we suspect that its binding to vWF may impede its interaction with the protease. The effect of VP-1 on the cleavage of BHK cell vWF mutant R834W is shown in Fig 7. Whereas VP-1 was inhibitory to the vWF cleavage, two other MoAbs M13 and M31 exhibited no such effect. The IC50 of VP-1 was approximately 0.3 μmol/L mouse IgG.
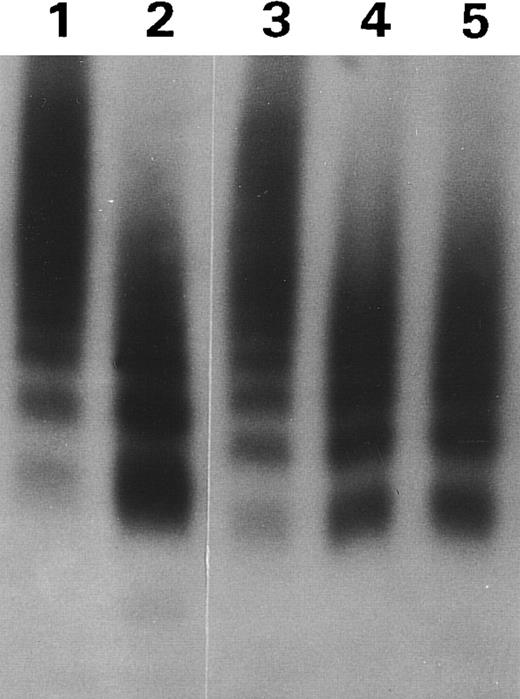
MoAb VP-1 decreased proteolytic cleavage of the R834W vWF mutant. Autoradiograph of a 1.5% SDS agarose gel showing the size distribution of the vWF mutant incubated with TBS (lane 1) or the plasma proteinase after treatment, respectively, with 1/10 TBS (lane 2), VP-1 (lane 3), M13 (lane 4), or M31 (lane 5).
MoAb VP-1 decreased proteolytic cleavage of the R834W vWF mutant. Autoradiograph of a 1.5% SDS agarose gel showing the size distribution of the vWF mutant incubated with TBS (lane 1) or the plasma proteinase after treatment, respectively, with 1/10 TBS (lane 2), VP-1 (lane 3), M13 (lane 4), or M31 (lane 5).
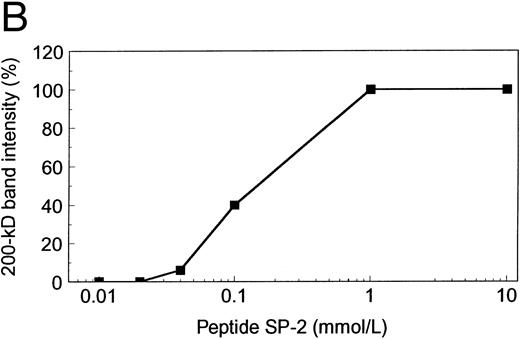
To establish the specificity of VP-1 inhibition, the antibody was treated with synthetic peptide SP-2 before incubation with the vWF mutant R834W (Fig 8A). SP-2 reversed the inhibition of vWF cleavage caused by VP-1. The reversal was dependent on the concentration of SP-2 (Fig 8B). When SP-2 concentration was increased to 0.1 mmol/L, vWF cleavage, as measured by 200-kD fragment generation, was restored to 40%. At 1 mmol/L SP-2, vWF cleavage inhibited by VP-1 was fully restored. SP-2 alone did not exhibit any inhibitory effect on vWF cleavage by the proteinase (data not shown).
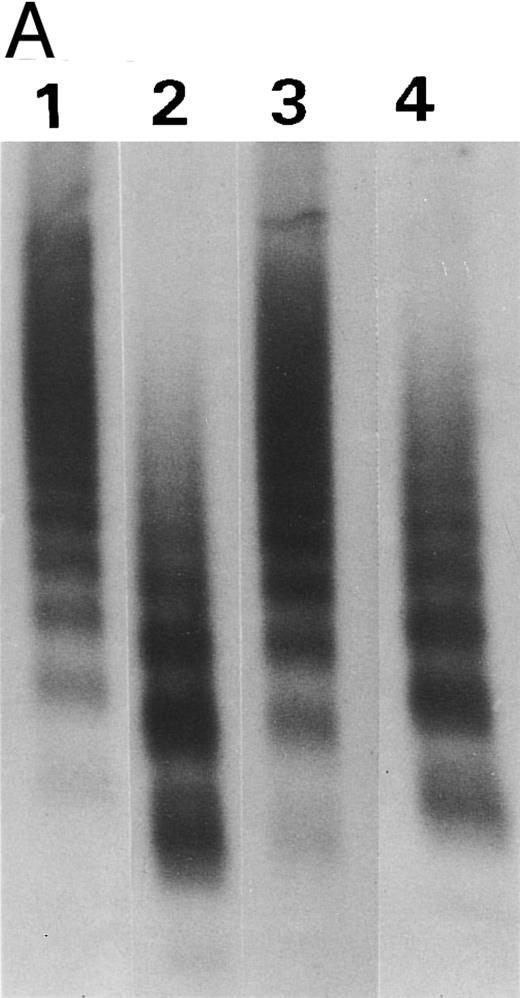
Synthetic peptide SP-2 (described in the Materials and Methods) reversed the inhibitory activity of antibody VP-1. (A) An autoradiograph of a 1.5% SDS agarose gel showing the size distribution of the R834W vWF mutant incubated with TBS (lane 1), the plasma proteinase (lane 2), the plasma proteinase in the presence of VP-1 (lane 3), and the plasma proteinase in the presence of VP-1 and 1 mmol/L SP-2. (B) Concentration dependence of SP-2 reversal of VP-1 inhibitory activity. VP-1 (90 μmol/L mouse IgG) treated with designated concentrations of SP-2 was added to the vWF mutant before the plasma proteinase. The intensity of the 200-kD band generated from the vWF was plotted against the SP-2 concentration on a log scale.
Synthetic peptide SP-2 (described in the Materials and Methods) reversed the inhibitory activity of antibody VP-1. (A) An autoradiograph of a 1.5% SDS agarose gel showing the size distribution of the R834W vWF mutant incubated with TBS (lane 1), the plasma proteinase (lane 2), the plasma proteinase in the presence of VP-1 (lane 3), and the plasma proteinase in the presence of VP-1 and 1 mmol/L SP-2. (B) Concentration dependence of SP-2 reversal of VP-1 inhibitory activity. VP-1 (90 μmol/L mouse IgG) treated with designated concentrations of SP-2 was added to the vWF mutant before the plasma proteinase. The intensity of the 200-kD band generated from the vWF was plotted against the SP-2 concentration on a log scale.
DISCUSSION
It has been suggested that the decrease of large and intermediate multimers in type 2A group 2 vWD results from excessive proteolysis of the vWF molecules. Recently, the presence of a plasma proteinase that causes physiologic cleavage of normal vWF has been identified.4,6 9 The present study investigates the proteolytic susceptibility of recombinant type 2A vWF and shows that two type 2A group 2 vWF mutants, R834W and R834Q, exhibited increased susceptibility to the plasma proteinase. Cleavage of each recombinant vWF generated proteolytic fragments that were indistinguishable from those found in normal or type 2A plasmas. Proteolytic cleavage was associated with a decrease of vWF ristocetin cofactor activity. Furthermore, we have also shown that the proteolysis can be inhibited by tetracyclines or MoAb VP-1.
Both R834W and R834Q vWF mutants were secreted from mammalian cells in a size distribution similar to that of WT vWF. Techniques of multimer analysis involving immunoisolation and/or immunoblotting tend to minimize the intensity of the large forms (H.-M.T., unpublished observation). This may explain why the size distribution of the vWF expressed in COS-7 cells was more weighted toward the large forms than previously described. Because normal vWF is secreted as an extra large polymer from either cultured endothelial cells1 or ex vivo microvascular preparations,2 interest for proteolytic susceptibility was focused on the large forms.
Apparently, processing of vWF in the COS-7 cells differed from that in the BHK cells. The conditions of the two expression systems were different. The concurrent expression of furin promoted cleavage of the propolypeptide in the BHK cells. The COS-7 cells culture media were collected in proteinase inhibitors, whereas the vWF expressed in BHK cells were affinity-purified. Although a direct comparison was not performed, the WT vWF expressed in COS-7 cells appeared to be more susceptible to the proteinase than that expressed in BHK cells. The causes of the difference are not determined. The multimer size distributions are different. The vWF in BHK cells contained relatively more intermediate multimers and less of the extra large forms. A higher sensitivity of the extra large forms to the proteinase than the large and intermediate multimers might account for the difference between COS-7 and BHK cell vWF. Alternatively, the presence of pro-vWF may also be a factor. When analyzed by SDS-PAGE under reducing conditions, the COS-7 cell's vWF contained approximately 60% pro-vWF and 40% mature vWF (data not shown). Because the small forms were significantly less than 50% of the total vWF, some pro-vWF was likely to be present in the large forms. The WT vWF expressed in BHK cells did not contain the propolypeptide and, as the plasma vWF, was only slightly susceptible to the proteinase in the absence of guanidine HCl. This finding raises the other intriguing possibility that the apparent sensitivity of the WT vWF in COS-7 cells to the proteinase was related to the presence of propolypeptide. Whether cleavage of the propolypeptide facilitates the folding of vWF into less susceptible conformation remains to be further investigated.
As determined by SDS-PAGE and the reactivity with MoAb VP-1, the 200-kD and 350-kD fragments generated from the vWF mutants were indistinguishable from those of the WT vWF treated with guanidine HCl or the dimers of the 140-kD and 176-kD fragments found in normal plasma.5 These observations suggest that cleavage of the vWF mutants occurred at the same T842/M843 peptide bond and that the amino acid substitutions caused by the mutations shifted the vWF molecules to more susceptible formations.
Compared with the WT vWF, neither of the vWF mutants gave rise to the 189-kD fragment generated from the WT vWF. It is interesting to note that the same 189-kD fragment found in normal plasma is also absent in the plasma of type 2A vWD.22 Presumably, the mutation makes the Y842/M843 peptide bond more susceptible to the proteinase, so that cleavage leading to the generation of the 189-kD fragment is minimized. Alternatively, because the 189-kD fragment cleavage site is on the amino terminal side of Y842/M8434 and may contain the substituted amino acid residue at position 834, any 189-kD fragment generated in the process may remain susceptible to the proteinase and undergo further cleavage at the Y842/M843 site to become the 176-kD fragment.
As with plasma or endothelial vWF,5 6 the recombinant vWF was cleaved by the plasma proteinase in a conformation-dependent manner. Thus, exposure of the recombinant vWF to guanidine HCl enhanced the proteolytic cleavage. The response of the vWF to guanidine HCl was biphasic. A similar biphasic response was also observed in purified plasma vWF (H.-M.T., unpublished data). The decrease in cleavage at higher concentrations of guanidine HCl was not due to its denaturing effect on the proteinase, because in separate experiments in which guanidine HCl was added before vWF, the cleavage was not different from that in which guanidine HCl was replaced by TBS. These data suggest that an intermediate, rather than completely unfolded, conformation of vWF was most susceptible to the proteinase and that the mutation shifts the domains of vWF molecules that interact with the proteinase toward the intermediate conformation. It is also possible that the proteinase interacted with vWF molecules at more than the cleavage site and that the other sites were also affected by the point mutation. This would explain the difference between the WT and the R834W mutant in the second-phase response to guanidine HCl. Future studies are needed to elucidate how the proteinase interacts with vWF molecules and how shear stress affects the proteolysis of type 2A vWF mutants.
Importantly, the vWF mutants are different in other aspects from normal vWF treated with guanidine HCl. Whereas guanidine HCl causes an irreversible inactivation of vWF ristocetin cofactor activity,6 the recombinant type 2A vWF mutants have normal or nearly normal activity.17 It is conceivable that the amino acid substitution caused a restricted conformational change, whereas the effect of guanidine HCl was more generalized. The kinetics of cleavage were also different. Although cleavage of vWF in the presence of guanidine HCl reached a plateau after 15 to 30 minutes, that of mutant vWF continued. The proteolytic susceptibility of vWF caused by guanidine HCl was partially reversible.6 It is possible that, because guanidine HCl was diluted in the reaction mixtures, the smaller forms generated from the WT vWF folded into a more resistant conformation. In contrast, the smaller forms generated from vWF mutant might remain susceptible to cleavage.
The plasma proteinase belongs to the metalloproteinase group because its activity, although not abolished by dialysis against buffers free of metallic cations, is inhibited by EDTA, EGTA, or 1,10-phenanthroline.6 The proteinase activity is not affected by other proteinase inhibitors such as phenylmethylsulfonyl fluoride, diisopropyl fluorophosphonate, leupeptin, N-ethyl maleimide, iodoacetamide, E64, and pepstatin A. A similar metal cation-dependent proteinase has also been partially purified and characterized by Furlan et al.10 Several members of the matrix metalloproteinase (MMP) family, including MMP-2 (gelatinase A), MMP-9 (gelatinase B), and a 65-kD collagenase are present in the plasma or serum.23-25 The majority of these proteinases are in latent forms and the small fraction that are activated are bound to proteinase inhibitors.26 As a result, matrix metalloproteinase activity is not detected by direct assays.25,26 The plasma vWF proteinase does not bind to gelatin-Sepharose6 and is different from the matrix metalloproteinase family5 in that it is active in the plasma and is not inhibited by batimastat, a peptide analogue of the collagen substrate that inhibits plasma matrix metalloproteinases with an IC50 less than 30 nmol/L.12 Despite these differences, the plasma vWF proteinase was similar to matrix metalloproteinases in that its activity was dependent on Ca2+ or Zn2+ and was inhibited by tetracyclines. The doxycycline IC50 for the proteinase is similar to that for MMP-1 (collagenase, IC50 = 280 to 300 μmol/L),27 but is much higher than those for MMP-8 (neutrophil collagenase) and MMP-9 (IC50 = 10 to 30 μmol/L and 30 to 50 μmol/L, respectively) or the plasma levels in patients receiving therapeutic doxycycline (5 to 10 μmol/L). Because inhibition persisted even after excess doxycycline was removed by dialysis, the inhibitory effect was directed against the proteinase rather than the vWF molecules.
The plasma proteinase used Ca2+ or Zn2+ for its activity because either of them, but not Cu2+ or Mg2+, reversed the EDTA inhibition. The inhibitory activity of doxycycline was partially blocked by low concentrations of Zn2+ or Cu2+. Attempts to completely block the inhibitory activity of doxycycline were limited by the inhibitory effect of high concentrations of Zn2+ or Cu2+ per se to the proteinase. Nevertheless, the data suggest that the inhibitory activity of doxycycline was related to its cation binding property. However, doxycycline inhibition of the vWF proteinase was different from that by EDTA, because the inhibition, even after the proteinase/doxycycline mixture was dialyzed to remove excess doxycycline, was not reversed by Ca2+ or Zn2+ at concentrations that restored the activity inhibited by EDTA.
The lack of effect on doxycycline by Ca2+ suggests that Zn2+ is a better candidate for the physiologic cation of the metalloproteinase. Alternatively, doxycycline might have better interaction with proteinase-bound Ca2+ than with free Ca2+. The physiologic cation of the proteinase need to be further investigated when the proteinase is purified. We speculate that doxycycline inhibits the proteinase by binding to it at the metallic cation active sites. The binding of doxycycline to the proteinase is further supported by the observation that the yellowish discoloration of the proteinase persisted despite extensive dialysis.
Although how the proteinase interacts with vWF molecules is not clear, it is possible that the amino acid residues surrounding the Tyr842/M843 cleavage site are critical for proteinase interaction. The inhibitory effect of VP-1 on vWF cleavage supports this hypothesis. We speculate that the IgG molecule bound to the surrounding amino acid sequence on the amino terminal side hinders the approach of the proteinase to its target peptide bond. Although data on only the recombinant R834W vWF mutant are presented, VP-1 also inhibited cleavage of the R834Q mutant as well as WT vWF treated with guanidine HCl. The inhibition of vWF cleavage by VP-1 was specifically related to its antibody activity because two other MoAbs M13 and M31 did not show any inhibitory activity and the inhibition was reversed by its epitopic synthetic peptide SP-2.
In summary, unlike WT vWF, recombinant type 2A group 2 mutants R834W and R834Q exist in susceptible conformations and are cleaved by the plasma metalloproteinase under conditions that cause little cleavage of normal vWF. It is conceivable that, in the circulation, the vWF mutants continue to be proteolyzed under shear stress conditions that are not sufficient to cause unremitting cleavage of normal vWF. This would explain why type 2A plasma vWF contains less large and intermediate multimers and is consistent with the observation that more of the large forms are found when type 2A blood is collected in EDTA,14 15 which prevents further cleavage in the test tubes. Because it is very likely that the bleeding diathesis of type 2A vWD is caused mainly by the lack of the large multimers, inhibition of the proteinase, leading to retention of the larger forms in the plasma, should correct the hemostatic defect. Another approach to prevent loss of the large multimers is to protect the vWF from the proteinase. Although neither tetracyclines or monoclonal VP-1 in the present forms would be suitable for clinical trials, the results of this study provide new directions for developing specific treatment of type 2A vWD.
ACKNOWLEDGMENT
The authors thank Dr Z.M. Ruggeri for kindly providing the MoAbs.
Address reprint requests to Han-Mou Tsai, MD, Division of Hematology, Montefiore Medical Center, 111 E 210th St, Bronx, NY 10467.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal