Abstract
The primate erythrocyte (E) complement receptor, CR1, is a transmembrane glycoprotein located in clusters on the surface of E. In vivo studies have demonstrated that during processing and clearance of complement-opsonized immune complexes, large amounts of immunoglobulin G (IgG) can be bound to primate E via CR1 with no E loss or lysis. However, when comparable amounts of IgG are bound to other sites on E, in many cases the E are cleared from the circulation by the mononuclear phagocytic system. Therefore, due to its role in immune complex processing, CR1 may represent a privileged site on the primate E. To delineate further this property of E CR1, we performed in vitro phagocytosis assays in the absence of complement and examined the ingestion of E, opsonized at various sites with IgG, by peripheral blood monocytes. When either human or rhesus monkey E were opsonized at sites other than CR1, with between 1,000 and 15,000 IgG per E, substantial phagocytosis of E was evident. However, when comparable amounts of IgG were bound exclusively via CR1, little, if any, phagocytosis was observed. The key to the low phagocytic level of E opsonized via CR1 may be related to the requirements of a “zipper mechanism” for phagocytosis first annunciated by Griffin et al. Based on their findings, we suggest that due to the presence of preexisting clusters of CR1 on the E membrane, large amounts of IgG can be bound to E under conditions that preclude circumferential engagement (and phagocytosis) of the entire E by Fc receptors on the monocyte.
THERE IS voluminous literature, based on models of immune hemolytic anemia, which demonstrate that binding of immunoglobulin G (IgG) to erythrocytes (E) can lead to hemolysis and/or uptake and destruction of the E by phagocytes.1-6 Whether the E are hemolyzed or taken up by macrophages and destroyed appears to depend on several factors, including the number of IgG bound per E, and the distribution of IgG on the E, which in turn can be influenced by the distribution of specific sites on the E at which the IgG is bound. For example, in vivo studies have demonstrated that during immune complex clearance, binding of complement (C3b)-opsonized immune complexes (containing large amounts of IgG) to primate E via the complement receptor CR1 (specific for C3b) leads to clearance of the immune complexes without E destruction.7,8 Emlen et al9,10 have performed comparable in vitro studies and have reported that E sensitized with C3b-opsonized immune complexes (bound to CR1) are also not phagocytosed by monocytes. These observations have been ascribed to the properties of CR1 in facilitating the E immune complex clearance mechanism.7 8
We have reported in vivo and in vitro studies that demonstrate that substantial amounts of IgG can be bound to primate E in the absence of complement by using chemically cross-linked monoclonal antibody (MoAb) constructs (heteropolymers) that contain anti-CR1 MoAbs cross-linked with MoAbs specific for other target antigens.11-16 The underlying assumption in this work is that an anti-CR1 MoAb bound to E CR1 serves as a surrogate for C3b, and that immune complexes bound to E in this model will be cleared from the circulation in a manner similar to that observed for the E immune complex clearance mechanism. In fact, in vivo experiments with heteropolymers indicate that IgG ligands complexed with antigens in immune complexes bound to E CR1 via anti-CR1 MoAbs are also cleared from the circulation of monkeys without causing any loss of E.12-16 Therefore, from the findings on both immune complex and heteropolymer-antigen clearance, primate E CR1 appears to have unusual properties in vivo that allow it to act as a privileged site in which specific binding of large amounts of IgG to the E can occur via CR1 without uptake and destruction of the E by phagocytic cells.12 Based on Emlen's reports, E containing IgG bound to CR1 in the absence of complement should also not be phagocytosed in vitro. The purpose of the present study was to test this hypothesis. We find that substantial amounts of IgG can be bound to human or rhesus monkey E via CR1 with little, if any, phagocytic uptake by human mononuclear cells. In contrast, and in agreement with numerous previous reports, when comparable amounts of IgG are bound to the E at several sites other than CR1, the E are taken up readily and phagocytosed.
MATERIALS AND METHODS
MoAbs and polyclonal antibodies.Purified 7G9 and 1B4, IgG2a and IgG1 mouse MoAbs specific for CR1, respectively, have been described.17 A polyclonal anti-CR1 antibody preparation was also obtained and purified from ascites fluid of a mouse immunized with soluble CR1.13 E3, an IgG1 MoAb specific for glycophorin A and B, was obtained as an ascites fluid preparation from Sigma (St Louis, MO). A9D3, an IgG2a MoAb specific for band 3 of rhesus monkey E,18 was generously provided by Dr D.H. Taylor at the National Institutes of Health, and streptavidin (SA) was purchased from Sigma. Affinity-purified rabbit antibodies to mouse IgG and fluorescein isothiocyanate (FITC)-labeled antimouse antibodies were obtained from Cappel (West Chester, PA). Purified MoAb HB 43, an IgG1 mouse MoAb specific for human IgG, MoAb 4-15, an IgG1 mouse MoAb specific for human IgE, and human IgG anti-Rho (D) antibodies have all been described.19-21 Several of the purified antibodies were labeled with 125I or were covalently modified with biotin, as described previously.11,13 22
Cells.E were obtained with informed consent from a variety of human donors, and E were obtained from sheep and several rhesus monkeys. E were stored as 25% dispersions in Alsever's and washed three times in phosphate-buffered saline containing 1% bovine serum albumin (BSA-PBS) before opsonization. In certain experiments, the E were biotinylated (see later). CR1 levels on the human E, determined by radioimmunoassay (RIA)14,22 with MoAb 7G9, varied between 300 and 600/E, and the E of the rhesus monkeys had approximately 3,000 CR1 epitopes per cell. Mononuclear cells were isolated from heparinized human blood by Ficoll-Hypaque centrifugation23-25 following the general procedures described by Edberg and Kimberly23 and were used without further stimulation. The isolated white blood cells typically contained 20% monocytes as determined by staining with myeloperoxidase. Cells were resuspended at a concentration of 10 × 106 cells/mL in RPMI with 20% fetal calf serum. More than 15 different human donors provided blood for the monocyte preparations; however, monocytes from a single donor were used on each experimental day.
Binding of antibodies to E.A variety of antibodies were bound to E by using a standardized sequence of incubation steps in which duplicate 100-μL aliquots of a 10% dispersion of E in BSA-PBS were incubated with equal volumes of diluted primary antibodies (some of which were 125I-labeled) for 15 minutes at 37°C (see Table 1 for complete definitions of all opsonization schemes). After three washes, those E samples opsonized with radiolabeled antibodies were counted to measure the amount of bound antibody and to calculate the number of IgG molecules bound per E.19 Subsequently, the E were reconstituted to 10% and incubated with the next reagent (usually 125I-labeled rabbit antimouse IgG). Following this step, all E pellets were counted, and the average number of IgG bound per E was calculated after correction for radioactivity bound to the E in the first incubation step. To confirm the stoichiometries reported for the experiments in which more than one opsonizing antibody was labeled with 125I, in control experiments E samples were coated with equal amounts of the same primary and secondary antibodies, but only the second antibody was 125I-labeled. Background (nonspecific) binding of the final developing reagent (ie, 125I-labeled rabbit antimouse IgG) to E that were not previously opsonized with mouse IgG was 10% or less of the values obtained for E that contained specifically bound mouse IgG. Dose-response experiments were conducted to determine the input levels of 125I-labeled rabbit antimouse IgG required for saturation of E-bound mouse IgG. Saturation of binding was defined by experiments in which further increases in inputs of 125I-labeled rabbit antimouse IgG did not significantly increase the amount of radiolabel specifically taken up by the E. In experiments in which saturation of binding was observed, incubation of 108 E with 4.3 μg/mL rabbit antimouse IgG led to saturation of binding. When ascites fluid preparations of mouse MoAbs were used, their E binding was verified by further development with 125I-labeled rabbit antibodies specific for the E-bound mouse MoAbs. In certain cases, multiple experiments are displayed for an averaged IgG/E ratio (see Figs 2 and 4, later) and reported stoichiometries (IgG bound per E) for individual points were within ±20% of the reported values.
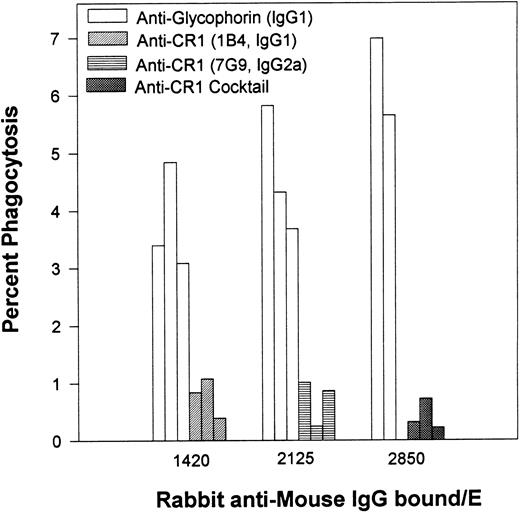
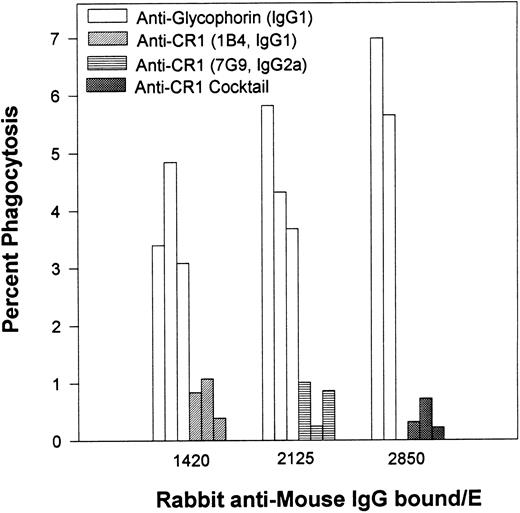
Percent of phagocytosis for human E opsonized with different inputs of an MoAb specific for glycophorin versus phagocytosis of E opsonized with anti-CR1 MoAbs. After opsonization with antiglycophorin or antiCR1 MoAbs, the E were then reacted with rabbit antimouse IgG. The multiple bars are representative of the same experiment performed on 3 different days.
Percent of phagocytosis for human E opsonized with different inputs of an MoAb specific for glycophorin versus phagocytosis of E opsonized with anti-CR1 MoAbs. After opsonization with antiglycophorin or antiCR1 MoAbs, the E were then reacted with rabbit antimouse IgG. The multiple bars are representative of the same experiment performed on 3 different days.
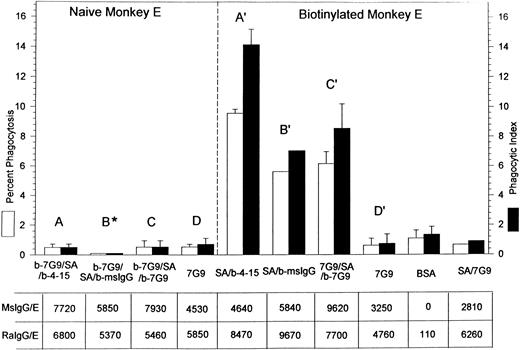
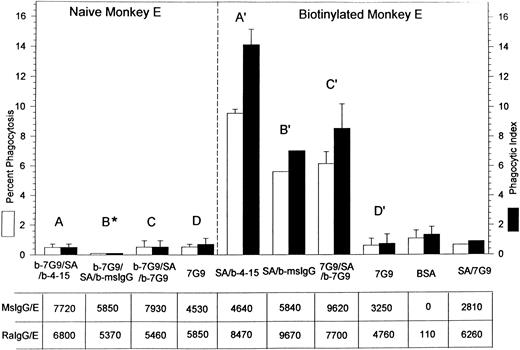
Percent of phagocytosis and phagocytic index of both naive and biotinylated rhesus monkey E containing bound mouse IgG and rabbit antimouse IgG. The E designations are the same as those displayed in Figs 1 through 3. The E in the B* bar represent naive E reacted with biotinylated antiCR1 MoAb 7G9 followed by SA followed by biotinylated mouse IgG; the B' designation denotes biotinylated E reacted with SA followed by biotinylated mouse IgG. The star by B indicates that the percent phagocytosis was very low (3 independent experiments) and the bars were included for clarity. The table displays the total number of mouse antibodies (MsIgG/E) and rabbit antimouse antibodies (RaIgG/E) bound per E. Results in Fig 4 represent different experiments from those in Fig 3. In most cases, the mean and SD for two or more independent experiments are given.
Percent of phagocytosis and phagocytic index of both naive and biotinylated rhesus monkey E containing bound mouse IgG and rabbit antimouse IgG. The E designations are the same as those displayed in Figs 1 through 3. The E in the B* bar represent naive E reacted with biotinylated antiCR1 MoAb 7G9 followed by SA followed by biotinylated mouse IgG; the B' designation denotes biotinylated E reacted with SA followed by biotinylated mouse IgG. The star by B indicates that the percent phagocytosis was very low (3 independent experiments) and the bars were included for clarity. The table displays the total number of mouse antibodies (MsIgG/E) and rabbit antimouse antibodies (RaIgG/E) bound per E. Results in Fig 4 represent different experiments from those in Fig 3. In most cases, the mean and SD for two or more independent experiments are given.
In experiments in which biotinylated E were used, the general opsonization procedures described by Edberg and Kimberly were followed.23 In brief, the amino groups of surface membrane proteins of E were first covalently labeled in phosphate-buffered saline with biotin using an N-hydroxysuccinimide biotin derivative.26 After three washes, streptavidin was bound to the E, and then biotinylated mouse MoAbs were bound to the E. In a final step, 125I-labeled rabbit antimouse IgG was bound. When any one or two parts of the opsonization steps were omitted (eg, E were not biotinylated, or SA and biotinylated MoAb were not added to the biotinylated E) the amount of 125I-rabbit antimouse IgG bound to the E decreased by more than a factor of 20 (eg, see Figs 3 and 4, later). Similar findings have been reported previously.26 All E pellets were resuspended in 1 mL of RPMI with 20% fetal calf serum (final hematocrit value, 1%) and were subjected to the phagocytosis assay.
Percent of phagocytosis of biotinylated rhesus monkey E containing bound mouse IgG in the absence (□) or presence (▨) of rabbit antimouse IgG. E contained IgG bound to CR1 (antiCR1 MoAb 7G9, mouse polyclonal anti-CR1 [p@CR1], and SA/p@CR1), or the E were treated with BSA-PBS followed by rabbit antimouse IgG (BSA). The third and fourth bars, respectively, represent biotinylated E treated consecutively with SA and biotinylated MoAb 7G9, or with nonbiotinylated MoAb 7G9 followed by SA and then biotinylated MoAb 7G9. The bar to the right of the dashed line represents the phagocytosis of biotinylated sheep E reacted with SA followed by biotinylated MoAb 7G9. The total number of rabbit antimouse IgG antibodies bound per E were (from left to right): 87, 4,000, 9,200, 5,300, 4,200, 4,300, 1,400.
Percent of phagocytosis of biotinylated rhesus monkey E containing bound mouse IgG in the absence (□) or presence (▨) of rabbit antimouse IgG. E contained IgG bound to CR1 (antiCR1 MoAb 7G9, mouse polyclonal anti-CR1 [p@CR1], and SA/p@CR1), or the E were treated with BSA-PBS followed by rabbit antimouse IgG (BSA). The third and fourth bars, respectively, represent biotinylated E treated consecutively with SA and biotinylated MoAb 7G9, or with nonbiotinylated MoAb 7G9 followed by SA and then biotinylated MoAb 7G9. The bar to the right of the dashed line represents the phagocytosis of biotinylated sheep E reacted with SA followed by biotinylated MoAb 7G9. The total number of rabbit antimouse IgG antibodies bound per E were (from left to right): 87, 4,000, 9,200, 5,300, 4,200, 4,300, 1,400.
Phagocytosis assay.Opsonized E were incubated with freshly isolated, unstimulated human monocytes following the procedures described by Edberg and Kimberly.23 Typically, 150 μL of the E suspension (1 × 108 E/mL) was mixed with an equal volume of monocytes (1 × 107 cells/mL), and after centrifugation at 1,500g for 5 minutes, samples of each mixture were subject to 1- and 2-hour incubations at 37°C. Results for the 2-hour incubations are reported, and similar but slightly lower levels of phagocytosis were observed at 1 hour. After the incubations, the noninternalized E were hypotonically lysed for 45 seconds with 1.5 mL of 0.1% NaCl. Subsequently, 1.5 mL of 1.8% NaCl was added, and after a brief centrifugation and wash step, the pelleted material (100 μL) was dispersed. The degree of phagocytosis was determined by examining between 200 and 500 mononuclear cells of the dispersion at 1,000-fold magnification under oil by light microscopy. Internalized E were counted by each of two observers in a blinded fashion, and phagocytic levels are reported based on the total number of monocytes counted. The percent phagocytosis is the number of mononuclear cells, per 100 counted, containing one or more internalized E. For positive samples, the difference between the percent phagocytosis measured by an individual observer and the reported value varied between 0% and 50% of the reported value. However, in no cases were there disagreements between observers with respect to identification of positive samples (phagocytosis > 3%) and negative samples (typically 1% ± 1% phagocytosis). The phagocytic index refers to the total number of internalized E counted per 100 mononuclear cells. Finally, fluorescence microscopy was performed on selected E samples. After mouse IgG was bound to E, the E were stained with saturating inputs of FITC-linked rabbit antimouse IgG and further processed following previously reported procedures,21 except in the final step, a drop of Cytoseal 60 mounting media (Stephens Scientific, Riverdale, NJ) was added to the slides.
RESULTS
Experiments with human E.Initially, varying amounts of polyclonal human IgG antibodies to the Rho (D) antigen were used to opsonize human E, since E opsonized with anti-Rho antibodies have often been used as a standard to detect and/or verify phagocytic activity of white blood cells.27-29 The E were then treated with mouse MoAb HB 43 (specific for human IgG and binding with a stoichiometry of 1:1)19 followed by saturating inputs of 125I-labeled rabbit antibodies to mouse IgG. Results from the phagocytosis assay indicate that these opsonized E were readily ingested by mononuclear cells in the present study (Fig 1). Alternatively, comparable amounts of rabbit IgG were bound to human E using several other approaches. In a parallel experiment, biotinylated MoAb 4-15 (mouse antihuman IgE) was attached to biotinylated E via a streptavidin linkage. Opsonization with MoAb 4-15 was once again followed by incubation with 125I-rabbit antimouse IgG, and these E (b-E/SA/b-4-15) were also readily phagocytosed. In contrast, the same biotinylated MoAb 4-15 was also bound to the E via CR1 using a biotinylated anti-CR1 MoAb (b-7G9) and streptavidin (E/b-7G9/SA/b-4-15). Although comparable amounts of rabbit IgG were bound per E, no phagocytosis was observed when all of the MoAb 4-15 was fixed specifically to CR1. Similarly, upon attachment of varying amounts of anti-CR1 MoAbs to the human E via CR1 along with saturating rabbit antimouse IgG, these E were also not phagocytosed (Fig 1).
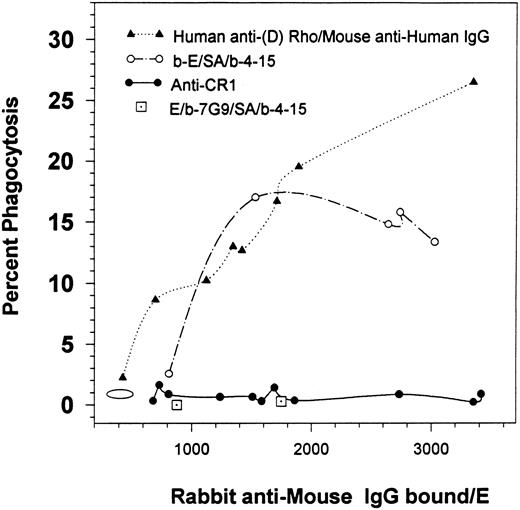
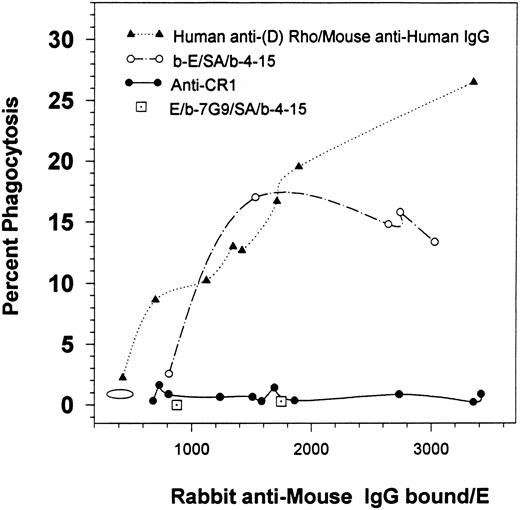
Percent of phagocytosis for human E containing varying amounts of bound rabbit IgG. E were consecutively opsonized with varying amounts of human anti-(D) Rho antibodies followed by a mouse MoAb (HB 43) specific for human IgG followed by rabbit antimouse IgG. Alternatively, the E were biotinylated (b-E), and then consecutively treated with SA, biotinylated mouse MoAb 4-15, and rabbit antimouse IgG. In the third model, the E were first opsonized with various anti-CR1 MoAbs followed by rabbit antimouse IgG. Finally, the two single points (⊡) represent nonbiotinylated E that were consecutively treated with biotinylated anti-CR1 MoAb 7G9, followed by SA, biotinylated MoAb 4-15, and finally by rabbit antimouse IgG. The graph portrays the results for multiple independent studies. The oval demonstrates the background level of phagocytosis for E treated with BSA-PBS followed by rabbit antimouse IgG. The solid circle at 2,732 IgG/E is also reported in Fig 2.
Percent of phagocytosis for human E containing varying amounts of bound rabbit IgG. E were consecutively opsonized with varying amounts of human anti-(D) Rho antibodies followed by a mouse MoAb (HB 43) specific for human IgG followed by rabbit antimouse IgG. Alternatively, the E were biotinylated (b-E), and then consecutively treated with SA, biotinylated mouse MoAb 4-15, and rabbit antimouse IgG. In the third model, the E were first opsonized with various anti-CR1 MoAbs followed by rabbit antimouse IgG. Finally, the two single points (⊡) represent nonbiotinylated E that were consecutively treated with biotinylated anti-CR1 MoAb 7G9, followed by SA, biotinylated MoAb 4-15, and finally by rabbit antimouse IgG. The graph portrays the results for multiple independent studies. The oval demonstrates the background level of phagocytosis for E treated with BSA-PBS followed by rabbit antimouse IgG. The solid circle at 2,732 IgG/E is also reported in Fig 2.
At saturation, between 300 and 600 anti-CR1 MoAbs bind to human E CR1.11 17 Therefore, the results for the anti-CR1 curve in Fig 1 indicate that multiple rabbit antimouse IgG were bound per anti-CR1 mouse MoAb. A variety of antibodies were used in the first opsonization steps for the other samples (eg, human or mouse IgG) and as not all of the reagents used were radiolabeled, we could not determine if the stoichiometry of binding of the rabbit antimouse IgG antibodies, per mouse IgG bound, were different in some of the other E samples. However, the other opsonization schemes in Fig 1 were designed to lead to the same final level of binding of rabbit antimouse IgG per E, in order to allow for a comparison of phagocytic levels of E containing the same amounts of rabbit IgG.
In the next series of experiments (Fig 2), human E were opsonized with varying inputs of a mouse MoAb (IgG1 isotype) specific for glycophorin followed by saturating inputs of rabbit antibodies to the E-bound mouse IgG. The levels of phagocytosis in this system were compared in parallel experiments for human E opsonized with two different mouse anti-CR1 MoAbs (7G9 and 1B4) or with a cocktail containing both anti-CR1 MoAbs, followed by the rabbit antimouse antibodies. Binding of IgG to E via glycophorin was effective in facilitating phagocytosis; however, little, if any, phagocytosis was observed for E in which all the IgG was bound via CR1. Anti-CR1 MoAb 1B4 is the same isotype (IgG1) as the antiglycophorin MoAb, and MoAbs 1B4 and 7G9 (IgG2a) bind to separate and noncompeting sites on E CR1.17 Differences between E opsonized with anti-CR1 MoAbs versus those opsonized with the antiglycophorin MoAb were more pronounced when the phagocytic indices were measured. For example, for 1,420 IgG/E (Fig 2), the average phagocytic index was 0.7 ± 0.2 for MoAb 1B4, and 5.4 ± 1.4 for the antiglycophorin MoAb.
Experiments with monkey E.Rhesus monkey E have, on average, sixfold more CR1 than human E; therefore, studies were extended to rhesus monkey E to examine cells with a higher level of bound IgG than could be achieved with human E (Table 2). E were treated with anti-CR1 MoAb 7G9 or with varying inputs of a MoAb specific for band 3. Saturating amounts of 125I-labeled rabbit antimouse IgG were added to each sample and ingestion of the E was compared in the phagocytosis assay. Although small variations in the background level of phagocytosis were evident in separate experiments (E not treated with mouse MoAb), there was a substantially lower level of phagocytosis for E opsonized via CR1 compared with E opsonized at band 3. Even when considerably less rabbit IgG was bound to E treated with the anti–band 3 MoAb (3,400 to 8,400 IgG per E compared with 9,300 to 13,900 for the anti-CR1 MoAb), the differences in phagocytic levels between the two samples are pronounced (Table 2).
As reported earlier in some of the experiments with human E, monkey E were biotinylated in order to use the streptavidin-biotin system to bind mouse IgG to sites on the monkey E other than CR1 or band 3. We compared the levels of phagocytosis for biotinylated monkey E containing mouse IgG (followed by rabbit antimouse IgG) bound either via CR1 or to alternative sites on the E (Fig 3). The third bar of Fig 3 demonstrates that when biotinylated anti-CR1 MoAb 7G9 was bound to the biotinylated E after treatment with streptavidin, a substantial level of E phagocytosis resulted. However, partitioning of biotinylated MoAb 7G9 between CR1 and streptavidin bound to biotinylated sites on the E may have occurred in this experiment. This question was addressed in several additional experiments. The fourth bar in Fig 3 represents biotinylated E that were first treated with naive MoAb 7G9 (to block CR1 sites on the E) and then streptavidin followed by biotinylated MoAb 7G9. The results below demonstrate that anti-CR1 MoAb 7G9 binds to clusters of E CR1. However, the biotinylated MoAb 7G9, attached to biotinylated E via streptavidin, should be bound in a more uniform and disperse manner. We found that E opsonized with biotinylated MoAb 7G9 at sites other than CR1 also experienced substantial phagocytosis. In contrast, the level of phagocytosis was quite low (comparable to background) whenever nonbiotinylated anti-CR1 MoAb 7G9 or polyclonal anti-CR1 antibodies (in the presence or absence of SA) were bound to the biotinylated E (7G9, or p@CR1, respectively, Fig 3), which is in agreement with our findings for human E. Finally, the question of partitioning of anti-CR1 MoAb 7G9 between CR1 and other sites on the E can be further resolved by examining sheep E, which lack CR1. Attachment of biotinylated anti-CR1 MoAb 7G9 to biotinylated sheep E via streptavidin also facilitates phagocytosis (Fig 3, last bar, on the right of the dashed line).
A final series of phagocytosis experiments was performed on either naive or biotinylated monkey E to address further quantitative questions regarding phagocytosis of E containing roughly comparable amounts of mouse IgG (followed by rabbit antimouse IgG) bound to either CR1 or to other sites on the E (Fig 4). Three isotypes of biotinylated mouse MoAbs were used for sensitization: IgG1 (MoAb 4-15), IgG2a (MoAb 7G9), and polyclonal mouse IgG. Monocyte Fc receptors are known to have varying affinities for different mouse antibody isotypes6; therefore, rabbit IgG was added to the E to try to achieve a more uniform level of opsonization of the E. Saturating inputs of rabbit IgG were not always used, since the goal of the experiments was to generate E with approximately equivalent final levels of E-bound rabbit antibodies to mouse IgG. However, in all experiments, the input concentration of rabbit antimouse IgG was identical for samples treated with anti-CR1 MoAbs versus those opsonized at sites other than CR1.
Figure 4 indicates that the location of antibody binding appears to be the deciding factor as to whether the E are phagocytosed. The first three pairs of bars (A, B*, C) on the left in Fig 4 show a low level of phagocytosis for naive E containing three biotinylated antibodies (4-15, polyclonal mouse IgG [msIgG], and 7G9) of three different isotypes. These biotinylated antibodies, and then rabbit antimouse IgG, were bound to CR1 by prior treatment of the E with biotinylated anti-CR1 MoAb 7G9 followed by streptavidin. In contrast, a much higher level of phagocytosis was evident (A′, B′, C′) when these same biotinylated antibodies (and rabbit antimouse IgG) were bound to biotinylated E at sites other than CR1. Control studies with anti-CR1 MoAb 7G9 bound to both naive and biotinylated E (D and D′) via CR1 also displayed a low level of phagocytosis, which remained low even if the latter E were also reacted with streptavidin (last bar on the right).
The amount of rabbit antimouse IgG that could be bound to the E tended to be greater for those E in which the mouse IgG was bound to sites on the E other than CR1 (7,700 to 9,670 IgG/E). The lower levels of rabbit IgG bound to CR1 (5,370 to 6,800 IgG/E) may simply be due to a packing phenomenon. In other words, as the mouse IgG is concentrated on CR1, rather than dispersed throughout the E, not all mouse IgG antibody may be equally recognized and bound by the rabbit antimouse IgG. However, the total amount of IgG bound to the E for A-C versus A′-C′ was similar, yet only the latter group showed substantial phagocytosis. Finally, we also examined phagocytosis of selected samples that were not reacted with rabbit antimouse IgG and simply contained the mouse IgG at the levels displayed in Fig 4, and the same trends were evident. For example, after opsonization with mouse IgG alone, B* (5,850 IgG/E) showed less than 1% phagocytosis (three independent experiments), while the results for B′ (5,840 IgG/E) after opsonization with mouse IgG alone yielded 6% ± 0.5% phagocytosis (two separate experiments).
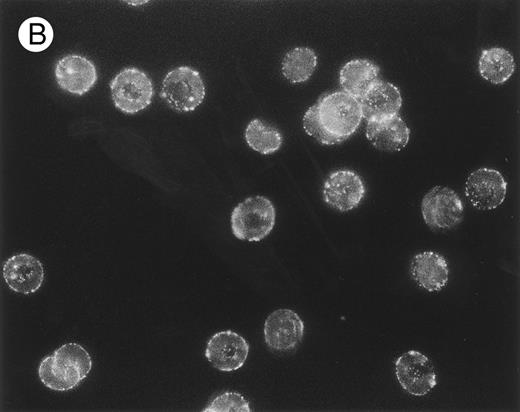
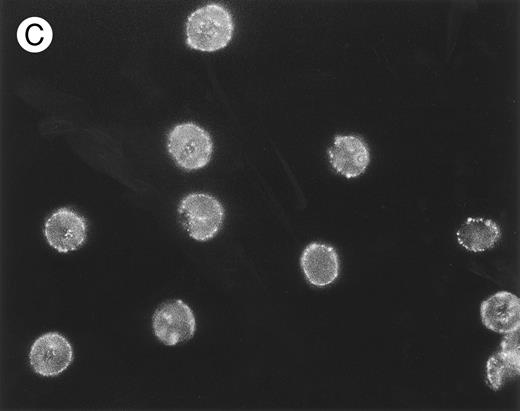
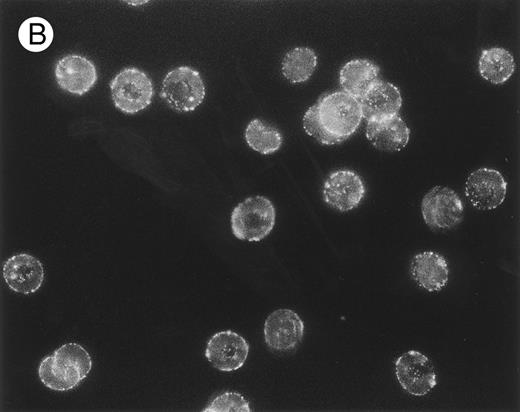
The packing and distribution of bound IgG antibodies likely plays a major role in determining whether an E is recognized and phagocytosed. Therefore, we performed a series of fluorescence microscopy experiments on selected E samples (Fig 5), which had been sensitized with approximately equivalent levels of mouse IgG as used in Table 2. The anti-CR1 MoAb 7G9 clearly appears bound in clusters to monkey E, and there is a marked heterogeneity in the fluorescence intensity between individual E. These findings are in agreement with several reports that have documented the clustered nature and heterogeneous distribution of CR1 on human E.21,30,31 However, the distribution of bound mouse IgG on either monkey E treated with the anti–band 3 MoAb or on biotinylated monkey E reacted with streptavidin followed by biotinylated mouse IgG is quite different. In these latter two cases, the opsonizing IgG is bound in a diffuse and more homogeneous distribution. The distribution of fluorescence intensity on the biotinylated E is consistent with reports that multiple sites on these E can be opsonized with IgG using the streptavidin-biotin system.23,26 32
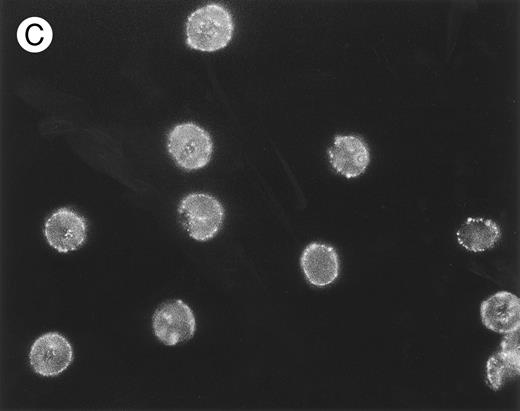
Localization, by immunofluorescence, of CR1, band 3 antigen, and biotinylated sites on E surface. E were incubated with (A) MoAb 7G9 (anti-CR1) or (B) MoAb A9D3 (anti–band 3) followed by FITC–rabbit antimouse IgG and prepared for immunofluorescence.
(C) Biotinylated E were incubated consecutively with SA, biotinylated mouse IgG, and FITC–rabbit antimouse IgG. Parallel RIA (not shown) of E samples treated with 125I-labeled rabbit antimouse IgG (instead of the FITC conjugate) showed binding of approximately 10,000 rabbit IgG per E for all three samples.
Localization, by immunofluorescence, of CR1, band 3 antigen, and biotinylated sites on E surface. E were incubated with (A) MoAb 7G9 (anti-CR1) or (B) MoAb A9D3 (anti–band 3) followed by FITC–rabbit antimouse IgG and prepared for immunofluorescence.
(C) Biotinylated E were incubated consecutively with SA, biotinylated mouse IgG, and FITC–rabbit antimouse IgG. Parallel RIA (not shown) of E samples treated with 125I-labeled rabbit antimouse IgG (instead of the FITC conjugate) showed binding of approximately 10,000 rabbit IgG per E for all three samples.
DISCUSSION
The present findings demonstrate that binding of moderate amounts of IgG from several different sources (human, mouse, and rabbit) to E (1,000 to 15,000 IgG/E) generally leads to recognition and phagocytosis of the E by mononuclear cells. However, E containing equivalent amounts of IgG bound exclusively via CR1 (using anti-CR1 MoAbs in the first opsonization step) stimulate little or no phagocytosis (Figs 1-4 and Table 2). These results cannot be due to a special property or isotype of the anti-CR1 MoAbs, since anti-CR1 MoAb 7G9 could induce phagocytosis of sheep or monkey E when it was bound to these cells at sites other than CR1 (Figs 3 and 4). In addition, the antiglycophorin MoAb that did induce phagocytosis was the same isotype as anti-CR1 MoAb 1B4, which did not facilitate phagocytosis (Fig 2). We did not observe any phagocytosis when a polyclonal anti-CR1 preparation was used to opsonize the E. Finally, all results were obtained with freshly isolated monocytes that were not treated with cytokines to stimulate upregulation of Fc receptors.33
The current results also provide further evidence that even when phagocytosis is evident, the distribution of sites on the E containing the opsonizing IgG may substantially influence the quantitative results. For example, at comparable levels of rabbit antimouse IgG bound (1,400 IgG/E), human E opsonized via the Rho (D) antigen are phagocytosed to a greater extent than human E opsonized via glycophorin. This effect may be due to a substantial difference in the distributions of IgG and distances between IgG bound to the respective antigens on the E.1-6 When biotinylated IgG is attached to the biotinylated E via streptavidin, the amount of phagocytosis appears to decrease slightly at the highest levels of opsonization (Fig 1). This result may be related to the accessibility of the E-bound mouse IgG to the rabbit antibodies. Since biotin can be covalently bound to many different locations on both an antibody and E, attachment of IgG to the E with the biotin-streptavidin system may generate a rather complex distribution of mouse IgG orientations on the E surface. The ability of the rabbit IgG to bind to this distributed mouse IgG and effectively opsonize E could be equally complex. We suggest that at the highest levels of opsonization, some of the bound IgG (mouse or rabbit) could actually block access of monocyte Fc receptors to other rabbit antibodies bound to the E. The differences in phagocytosis at the highest levels of opsonization may also relate to the choice of monocyte donor, since there was a difference in the proportion of monocytes between individuals, although the average was 20%. The fourth bar of Fig 3 indicates that additional binding of rabbit antimouse IgG did not substantially augment phagocytosis. This result (and similar findings in Fig 4 for B′) may again be related to how effectively the last layer of bound rabbit antibodies can bind to the biotinylated MoAbs and how effectively each of the antibodies can be recognized by the monocyte Fcγ receptors.
Although the results of this study can not necessarily be generalized, our findings suggest that binding of IgG to many sites on the E surface will lead to phagocytosis. Certainly, the Rho (D) antigen, glycophorin, and band 3 serve as effective sites for opsonization with IgG. Studies with biotinylated E, in which biotin is covalently attached to random proteins on the E membrane,23,26 32 suggest that a variety of other sites on the E can take up IgG and also mark the cells for phagocytosis. Fluorescence microscopy experiments provide further evidence that the distribution of opsonizing IgG on the E plays a role in deciding their phagocytic fate (Fig 5). The mouse IgG bound to the E (and developing FITC antimouse IgG) are clustered when the mouse IgG is fixed to CR1, yet a considerably more uniform, dispersed distribution is evident when either opsonized biotinylated E or E reacted with anti–band 3 MoAb are examined.
Although there may be other privileged sites on the E, the present studies confirm and extend previous in vivo findings. That is, relatively large amounts of IgG can be bound to E via CR1 without causing in vivo or in vitro lysis or phagocytosis of the E. Primate E CR1 possesses many unusual biophysical and immunologic properties that are consistent with these results. This receptor is at relatively low density on primate E.13,14,22 CR1 apparently sticks out of the E in a relatively rigid structure that resembles beads on a string,34 and it is also organized in clusters, rather than being monodisperse on the E surface.21,30,31,35 Large amounts of IgG can be attached and later removed from E CR1 in vivo. In the natural process C3b-opsonized immune complexes become bound to E CR1 and are then cleared from the circulation with no lysis or phagocytosis of the E.7,8 Anti-CR1 MoAbs can serve as surrogates for C3b, and a variety of cross-linking techniques allow attachment of large amounts of IgG to the E via CR1. E-bound IgG can be cleared from the circulation with no E loss by a mechanism that appears to be similar to the E-immune complex clearance reaction.12-17
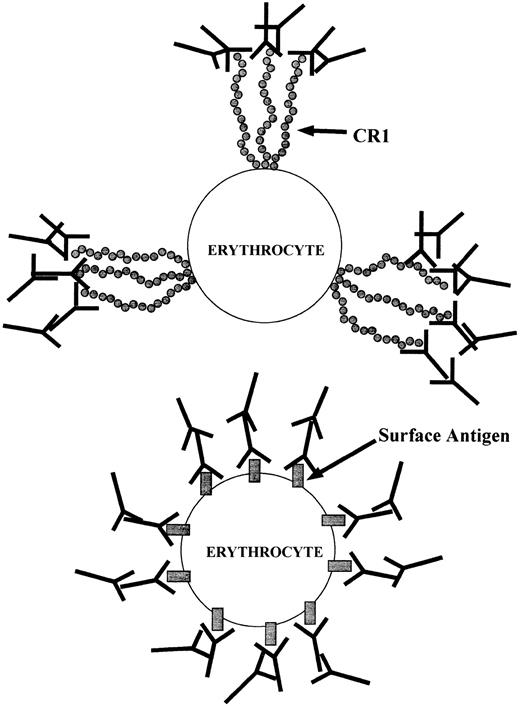
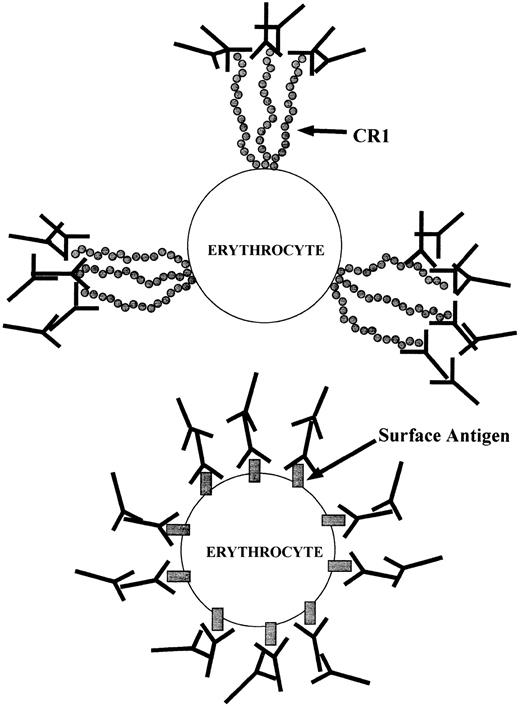
Schematic of IgG association with E. (Top) Anti-CR1 mouse IgG bound to CR1 clusters on the E surface, followed by attachment of rabbit antimouse IgG. (Bottom) Mouse IgG bound to an antigen dispersed throughout the E surface, followed by rabbit antimouse IgG.
Schematic of IgG association with E. (Top) Anti-CR1 mouse IgG bound to CR1 clusters on the E surface, followed by attachment of rabbit antimouse IgG. (Bottom) Mouse IgG bound to an antigen dispersed throughout the E surface, followed by rabbit antimouse IgG.
The key to the privileged site nature of CR1 and the fact that E opsonized via CR1 are not phagocytosed may be related to how IgG is recognized by phagocyte Fc receptors when it is bound to a cellular substrate. Griffin et al36,37 have demonstrated that a “zipper mechanism” is required for macrophages to ingest IgG-sensitized cells. That is, “ingestion of a particle requires the circumferential interaction of phagocytic receptors” (ie, Fc) “with particle bound ligands” (ie, IgG) “not involved in the initial attachment of the particle to the phagocytic cell.”37 In other words, there must be distribution of IgG ligands over almost the entire surface of the IgG-opsonized substrate cell for the phagocyte to surround and ingest the target cell. Griffin et al also reported that cells containing large amounts of aggregated IgG located in a cap were not phagocytosed, whereas the cells containing equivalent amounts of IgG bound in random positions over the entire surface of the cell were ingested.36,37 The low copy number for E CR1 and the fact that it is organized in clusters allows binding of relatively large amounts of IgG to the E either via C3b-opsonized immune complexes or through the use of anti-CR1 MoAbs as C3b surrogates. We suggest that since CR1 bound IgG is focused to a small number of points on the E, these conditions spare the E from phagocytosis by precluding operation of the zipper mechanism. The schematic in Fig 6 illustrates the basic idea. Although an equal amount of IgG is bound to the E in both cases, only the E in the bottom panel would be phagocytosed. As noted earlier, Emlen et al have examined the solution phase transfer of C3b-opsonized immune complexes from E to monocytes, and they have reported that in no case could any evidence be found for phagocytosis of the E during this process.9,10 There are, to our knowledge, no studies that specifically characterize the interaction of the Fc portion of human IgG, bound to E CR1, with Fcγ receptors on mononuclear cells. However, in addition to Emlen's studies, Kimberly et al have provided indirect evidence for the involvement of Fcγ receptors in immune complex clearance in vivo by demonstrating inhibition of clearance upon infusion of an MoAb that blocks IgG binding to FcγRIII.38
In the present study, we did not examine the fate of IgG ligands bound to E CR1. We have demonstrated in vivo the removal of these ligands from E in the liver and their phagocytosis, presumably by fixed tissue macrophages (Kupffer cells).12-15 Future in vitro studies with monocytes derived to adherent macrophages will attempt to model the in vivo process and examine the fate of IgG ligands bound to E via CR1. However, the results of the experiments described herein provide strong evidence that large amounts of IgG can indeed be bound to primate E via CR1 without causing the E to be internalized by mononuclear cells. This finding is certainly consistent with the apparent function of primate E CR1, which is to facilitate safe and rapid binding and clearance from the circulation of certain C3b-opsonized immune complexes without E destruction.7,8 The fact that large amounts of IgG can indeed be bound safely to the E via the C3b-surrogate anti-CR1 MoAbs provides additional evidence for the potential use of E CR1 in conjunction with bispecific methodologies for the treatment of diseases in which the eventual goal is to facilitate the safe and rapid clearance of pathogens from the circulation.11
ACKNOWLEDGMENT
We thank Dr Jeffrey Edberg and Dana Lau of the Hospital for Special Surgery for generously instructing us in phagocytosis assay methodology.
Supported by National Institutes of Health Grant AR43307; and by the Defense Advanced Research Projects Agency (DARPA), Director's Office, “Clearance of Pathogens,” DARPA Order No. MDA972-96-K-0001, P0001, issued by DARPA/CMO under Contract No. MDA972-96-K-0003.
Address reprint requests to Ronald P. Taylor, PhD, Department of Biochemistry, University of Virginia School of Medicine, Charlottesville, VA 22908.

![Fig. 3. Percent of phagocytosis of biotinylated rhesus monkey E containing bound mouse IgG in the absence (□) or presence (▨) of rabbit antimouse IgG. E contained IgG bound to CR1 (antiCR1 MoAb 7G9, mouse polyclonal anti-CR1 [p@CR1], and SA/p@CR1), or the E were treated with BSA-PBS followed by rabbit antimouse IgG (BSA). The third and fourth bars, respectively, represent biotinylated E treated consecutively with SA and biotinylated MoAb 7G9, or with nonbiotinylated MoAb 7G9 followed by SA and then biotinylated MoAb 7G9. The bar to the right of the dashed line represents the phagocytosis of biotinylated sheep E reacted with SA followed by biotinylated MoAb 7G9. The total number of rabbit antimouse IgG antibodies bound per E were (from left to right): 87, 4,000, 9,200, 5,300, 4,200, 4,300, 1,400.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.1068/8/m_bl_0015f3.jpeg?Expires=1765904410&Signature=siZVZEVwAR3RbnCaR7Cb38YmGeiwJM6v-AEir7FeGReHAH7EomfbM1fV9vjsub1WOAXzrKl-8-RlAhtpaL0VAx1qhhSNBYIRaAxiRiTAIxsGJSLHx43cdd1PHJQuWx2TCvy6av~qAiYDnp~XpRYVZRzi3mcbW1BcDyWarxs18dTZGE2--f-jvpkhxTTqx4Imi6uY2splTSjmObWmFcf-YqdcHOeZKt4atRNcYqpgBauZmnlqabtavprR8Uj8FdlGCmPk3kwKVTBzQDZUKWeCbsr0JSP-4K4qVttsoGtCi4nLfpHb083LTkPF2JcPmqKpIx4fRJEseEw-GtmxPSQeTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. Percent of phagocytosis of biotinylated rhesus monkey E containing bound mouse IgG in the absence (□) or presence (▨) of rabbit antimouse IgG. E contained IgG bound to CR1 (antiCR1 MoAb 7G9, mouse polyclonal anti-CR1 [p@CR1], and SA/p@CR1), or the E were treated with BSA-PBS followed by rabbit antimouse IgG (BSA). The third and fourth bars, respectively, represent biotinylated E treated consecutively with SA and biotinylated MoAb 7G9, or with nonbiotinylated MoAb 7G9 followed by SA and then biotinylated MoAb 7G9. The bar to the right of the dashed line represents the phagocytosis of biotinylated sheep E reacted with SA followed by biotinylated MoAb 7G9. The total number of rabbit antimouse IgG antibodies bound per E were (from left to right): 87, 4,000, 9,200, 5,300, 4,200, 4,300, 1,400.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/3/10.1182_blood.v89.3.1068/8/m_bl_0015f3.jpeg?Expires=1765904411&Signature=Vj5O0RxLZYY70ZqXDD07VBr2HnXGNY7yhjzY2dACpXGjyGgGCsh5qKkOaFxfsge2m4Qj5ui0Yp-F9O22Ljwm6HYeU5eLS6P4ePEPlQdz4M-V6W0hsnnL~iibttxLTt9aEL9O56SiDFXOSgMZigGV9revMZixkCPRd-dJSrm6kxEzNwr9c5M20PNi8pu7H9Z3Ft4irvakO9nKOLNN7RtR9haNHCuFDrrJPjTPyOAPSijJ1P9atuYOlhLw19yrWKB1CdvkKpmz7u5qXNWuJnyR9J9hnhFw0W3XoQMt9fXRXyzBdPdTHCn~t21o2vm1jJ-uQMWWRuAMNeSAzuVTRb4U-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)