Abstract
Recent studies in Western European populations have shown that peripheral T-cell non-Hodgkin's lymphomas (T-NHLs) are associated with Epstein-Barr virus (EBV) in a higher percentage than sporadic B-cell NHL (B-NHLs), and that the frequency of EBV-positivity might be influenced by the primary site of the tumor. Because of the geographic differences in EBV expression in Burkitt's lymphoma (BL) and Hodgkin's disease (HD), and the lack of studies of sporadic NHL from developing countries, we decided to survey the presence of EBV in a series of primary intestinal lymphomas from patients in Mexico and in Western Europe, and to analyze whether EBV status is influenced by tumor phenotype, and geographic or ethnic determinants. Paraffin-embedded tissue from 43 primary intestinal NHLs (19 cases from Mexico and 24 from Western Europe) were examined, including 17 high grade B-NHLs, 9 low grade B-NHLs, and 17 T-NHLs; 6 of which were enteropathy associated T-cell lymphomas. The distribution of histologic subtypes was similar in both groups. The presence of EBV was investigated with a combined approach using a nested polymerase chain reaction technique as well as immunohistochemistry for latent membrane protein-1 and in situ hybridization for EBV early RNA transcripts (EBER 1/2) RNAs. The median age of the Mexican patients was significantly lower than the median age of the European patients (32 v 62 years). This difference was most pronounced in patients with T-cell lymphoma (24 v 63 years). EBER-positive tumor cells were detected in 13 of the 43 (30%) cases of primary intestinal lymphoma, including 5 of 26 sporadic B-NHL (3 high grade and 2 low grade), and 8 of 17 T-NHL, all of which were classified as pleomorphic, medium and large cell. The rates of EBV-positivity were markedly different for European and Mexican cases. Whereas 7 of 7 (100%) T-NHL and 5 of 12 (42%) sporadic B-NHL of Mexican origin were EBER-positive, only 1 of 10 T-NHL and 0 of 14 sporadic B-NHL from Europe showed EBER expression in tumor cells. Latent membrane protein was positive in only 2 of 43 cases, 1 of which was an EBER-negative high grade B-NHL from Mexico that showed intact total mRNA in control hybridization. CD30 expression was found in 4 of 8 EBV-positive T-NHL and in none of the EBV-positive B-NHL. In contrast to European cases, intestinal NHLs from Mexico show a very high frequency of EBV-positivity, which is not limited to T-NHL, but includes a significant proportion of B-NHL. This study strongly suggests that similar to HD and probably BL, there are important epidemiologic differences in EBV association in intestinal T-cell NHL between European and Mexican populations. These differences might be the result of environmental factors, for example, earlier contact with childhood viruses on intestinal lymphomagenesis.
EPSTEIN-BARR VIRUS (EBV) has long been implicated in the pathogenesis of endemic Burkitt's lymphoma in Africa and undifferentiated nasopharyngeal carcinoma,1,2 and more recently has been found in a significant percentage of cases of Hodgkin's disease (HD).3-5 EBV is also associated with some high grade B-cell non-Hodgkin's lymphomas (NHL) and lymphoproliferative disorders in immunosuppressed individuals, where it is found in virtually all posttransplant lymphomas and some acquired immunodeficiency syndrome (AIDS)-associated lymphomas, particularly those of the central nervous system.6,7 In contrast, EBV has been found only infrequently in sporadic B-cell NHL (B-NHL).8 9
Recent studies in Western populations have shown that T-cell NHL (T-NHL) are associated with EBV in a higher percentage than sporadic B-cell NHLs.8,9 This is a somewhat unexpected finding in the light of the well known B-cell tropism of EBV through the EBV-specific receptor CD21 (CR2), which is expressed on B cells and developing T cells but not on mature peripheral T cells.10,11 However, more recent data including the demonstration of EBV-positive T cells in normal lymphoid tissues suggest that the lineage specificity of EBV might be less restricted as previously thought.12
The frequency at which EBV can be detected in nonendemic lymphomas, especially of T-cell type, arising in patients without a well-defined immunodeficiency, is not only dependent on histologic subtype, but also is influenced by geographic or ethnic determinants and the primary site of the tumor. In extranodal T-NHL, however, recent evidence suggests that the occurrence of EBV is site restricted,13-15 and does not depend on histologic subtype as has been suggested by others.16
In this study, the presence of EBV in a group of primary intestinal lymphomas (T-cell lymphomas and sporadic B-cell lymphomas) from Mexico and Western Europe was surveyed to determine whether significant differences in EBV positivity could be observed in relation to histology of the tumor, and country of origin. EBV was detected by polymerase chain reaction (PCR) as well as RNA in situ hybridization (ISH) for EBV early RNA transcripts (EBERs) and immunohistochemistry for the presence of EBV latent membrane protein-1 (LMP-1).
MATERIALS AND METHODS
Case selection.Fifty-six cases of primary intestinal lymphoma were selected from the files of the Departments of Pathology of the Instituto Nacional de la Nutricion, Mexico City, Mexico; Innsbruck University Hospital, Innsbruck, Austria; and University of Würzburg, Würzburg, Germany. Only resection specimens with paraffin-embedded tissue available for molecular and immunohistochemical studies were included. All cases were classified according to the updated Kiel classification and the proposal for a revised European-American lymphoma classification.17 The term enteropathy-associated was used either with an established diagnosis of celiac disease or with the finding of histologic features of celiac disease in the bowel uninvolved by lymphoma. The term enteropathy associated T-cell lymphoma (EATCL)-like was used according to the criteria described by Chott et al.18 These neoplasms have the morphology of EATCL with intraepithelial lymphocytes exclusively within the tumor margins and show no clinical or histologic evidence of enteropathy. All cases included were previously immunophenotyped on paraffin sections and in part on frozen sections. The neoplasm was considered T cell in origin when it expressed one or more T-cell markers (CD2, CD3, CD5, CD7 on frozen sections or CD3, CD43, and CD45RO on paraffin-embedded tissue) and did not express B-cell markers or appear to be B cell in origin when it expressed one or more B-cell markers (CD19 and/or CD20 and L26 on paraffin-embedded tissue) and/or showed light chain restriction. In addition all cases were tested for CD30 (Ber-H2, Dakopatts, Kopenhagen, Denmark). The cases were reviewed by two of the authors (L.Q.-M. and F.F.) and only when considered necessary, additional immunohistochemical studies were performed.
DNA extraction and PCR analysis.DNA extraction and detection of EBV-specific genomic sequences by PCR were performed as described previously.19 Sections (3 to 5 μm) from paraffin blocks were taken, dewaxed, and digested with proteinase K (200 to 400 μg/mL) at 60°C overnight. DNA was extracted with phenol/chloroform, precipitated and resuspended in 100 μL of sterile water. The presence of amplifiable DNA was confirmed by amplification of a 268 bp fragment of the β-globin gene.20 EBV-specific sequences were detected with a nested PCR using two primer pairs amplifying a 335 bp (outers) and a 253 bp (inners) fragment of the internal repeat region (BamH1W fragment) of the EBV genome.21 Both first and second round products were detected by UV examination of ethidium bromide-stained agarose gels. The results were confirmed by dot blot hybridization of the second round products with an internal control oligonucleotide labeled with digoxigenin.19 Beginning with sectioning, the above procedure was repeated once with every block for confirmation of results. A paraffin-embedded tonsil specimen from a patient with infectious mononucleosis served as a positive control for both PCR and ISH.
ISH and immunohistochemistry.All cases positive for EBV by PCR, half of the EBV-negative cases, and all lymphomas that failed to show amplifiable DNA were subjected to ISH using fluorescein-labeled oligonucleotides complementary to EBER1/2 (Dakopatts) and were stained for LMP-1 by immunohistochemistry (clone CS.1-4, Dakopatts). The ISH procedure was carried out under RNAse-free conditions. The optimal concentration of proteinase K for the prehybridization digestion of the slides was determined individually to reduce the influence of different fixation protocols and was in the range of 10 to 100 μg/mL proteinase K at 37°C for 15 minutes. After overnight hybridization at 42°C and stringent washing, bound probe was detected with the alkaline phosphatase/anti-alkaline phosphatase technique and Fast Red or NBT/BCIP substrate for color development as previously described.19
Immunohistochemistry for LMP-1 was performed with our routine streptavidin-biotin technique after microwave pretreatment of slides. Double stainings for EBERs and B- and T-cell antigens were performed in all cases that showed less than 10% EBER-positive tumor cells. After staining with L26 or UCHL-1 (Dakopatts), using diamino-benzidine as a substrate, the slides were subjected to ISH as described above. For color development, NBT/BCIP substrate was used for better discrimination of the signals of immunohistochemistry and ISH. EBER-negative cases were tested by ISH for viability of total mRNA using a fluorescein-labeled poly-d(T) probe (Biogenex, San Ramon, CA).
RESULTS
Of the 56 cases, 13 (23.2%) were excluded from the final analysis. Eight cases were intestinal lymphomas in posttransplant patients and are the subject of another study. One case was reclassified as nonlymphoid neoplasm after additional studies, and four cases failed both to amplify for the β-globin gene fragment and were negative with both the EBER and the poly-d(T) probe in ISH and therefore were considered as not evaluable. The remaining 43 cases are the subject of this study. For analysis the cases were divided in three groups: group A, T-cell lymphomas (17 cases); group B, sporadic B-cell lymphomas high-grade (17 cases); and group C, sporadic B-cell lymphomas low-grade (9 cases).
Group A.Of the T-cell lymphomas, 10 cases were European patients, and 7 were Mexican patients. Cases were classified as pleomorphic medium and large cell (13 cases), pleomorphic small cell (3 cases), or anaplastic large cell (1 case). Six of these cases were EATCL, of which 4 cases were European patients and 2 were Mexican; 5 cases were EATCL-like, 4 European patients and 1 Mexican patient; and 6 cases were non-EATCL, 2 cases were European and 4 were Mexican.
Group B.Of the sporadic high grade B-cell lymphomas, 11 cases were European patients, and 6 cases were Mexican patients. Cases were classified as MALT high grade (2 cases), Burkitt's (5 cases), and diffuse large cell either centroblastic or immunoblastic (10 cases). Six of the diffuse large cell cases showed a population of large, irregular, and sometimes multinucleated bizarre cells resembling Reed-Sternberg cells with high mitotic index reminiscent of the polymorphic EBV-positive lymphomas described in immunodeficient patients with AIDS or after organ transplantation.
Group C.Of the sporadic low-grade B-cell lymphomas, 3 cases were European patients, and 6 cases were Mexican patients. Four of these cases corresponded to MALT low-grade lymphomas, 4 to follicular center cell lymphomas (3 follicular and 1 diffuse), and 1 to lymphomatous polyposis (mantle cell lymphoma).
Patient characteristics.The main demographic data of the patients are shown in Table 1. The median age of the Mexican patients was significantly lower than the median age of the European patients (32 v 62 years). This difference was most pronounced in patients with T-cell lymphoma (24 v 63 years), and Burkitt's lymphoma (4 v 30 years), and less marked in patients with other types of B-NHL (52 v 60 years). Localizations included 29 cases in the small bowel, 9 cases in the ileocecal region, 3 cases in the large bowel, 1 case in the appendix, and 1 case involving the small and large bowel. Four of the 17 (23.5%) T-cell lymphomas were multicentric involving different areas of jejunum and ileum, whereas 3 of 26 (11.5%) B-cell lymphomas were multicentric. Four of the seven cases of T-NHL in Mexican patients involved the right colon with extension to the ileocecal region. These four cases morphologically corresponded to non-EATCL of the pleomorphic medium and large cell type.
EBV-DNA detection by PCR.In addition to the cases excluded above, 13 of the remaining 43 specimens (30%) showed a lack of amplifiable DNA with the the β-globin gene primers. There was no difference in the percentage of cases with insufficient DNA from the three different institutions. In the remaining 30 cases, EBV-specific sequences were detected by nested PCR followed by control hybridization with the internal oligonucleotide probe in 11 of 18 (61%) European lymphomas and 8 of 12 (66.6%) Mexican cases. In all cases EBV-specific products were detected in both DNA samples resulting from the two parallel extraction procedures.
Detection of EBER transcripts and LMP-1 expression, and correlation with PCR results.EBER-positive tumor cells were present in 13 of 43 (30.2%) primary intestinal lymphomas, including 5 of 26 sporadic B-NHL (3 high grade and 2 low grade), and 8 of 17 T-NHL all of which were classified as pleomorphic, medium, and large cell. The prevalence of EBV-positivity was clearly dependent on the tumor type and the country of origin. Whereas 7 of 7 (100%) T-NHL, and 5 of 12 (42%) B-NHL of Mexican origin harbored EBER-positive tumor cells, only 1 of 10 T-NHL and 0 of 14 sporadic B-NHL from Europe showed EBER expression in tumor cells. Two of the five positive B-NHL of Mexican origin corresponded to Burkitt's type. In contrast, none of the three Burkitt's lymphomas of European origin harbored EBER-positive tumor cells.
The EBER-positive intestinal lymphomas are described in Table 2. The percentage of EBER-positive tumor cells varied in the different tumor types. In the EBV-positive Burkitt's lymphomas virtually all viable cells showed EBER expression (Fig 1). Areas of necrosis and pycnotic cells were negative for EBERs. The T-NHL exhibited a broad range of reactivity from 1% to 100% of the neoplastic population (Fig 2A and B). Five of eight T-NHL showed labeling of at least 50% of the tumor cell population with a diffuse and sometimes focal distribution of EBER-positive cells; of these cases, two corresponded to EATCL of Mexican origin, and three were non-EATCL. Three cases of T-NHLs and the three EBV-positive B-cell lymphomas (MALT high grade, MALT low grade, and follicular center cell lymphoma) other than Burkitt's type showed a very heterogeneous distribution of the signal, with accumulation of EBER-positive tumor cells, mainly blasts in small areas, whereas the remainder of the section was almost completely devoid of positive cells. The amount of EBER-positive cells in these cases was between 1% and 20% of the tumor cell population.
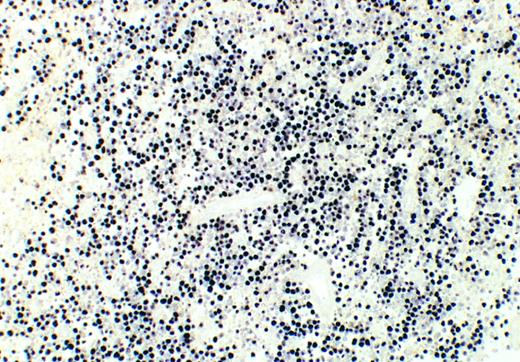
Mexican Burkitt's lymphoma. In situ hybridization reveals EBER1/2 transcripts in virtually all viable tumor cells. Necrotic cells are unstained. Case no. 9, original magnification ×100. Color development with NBT/BCIP substrate.
Mexican Burkitt's lymphoma. In situ hybridization reveals EBER1/2 transcripts in virtually all viable tumor cells. Necrotic cells are unstained. Case no. 9, original magnification ×100. Color development with NBT/BCIP substrate.
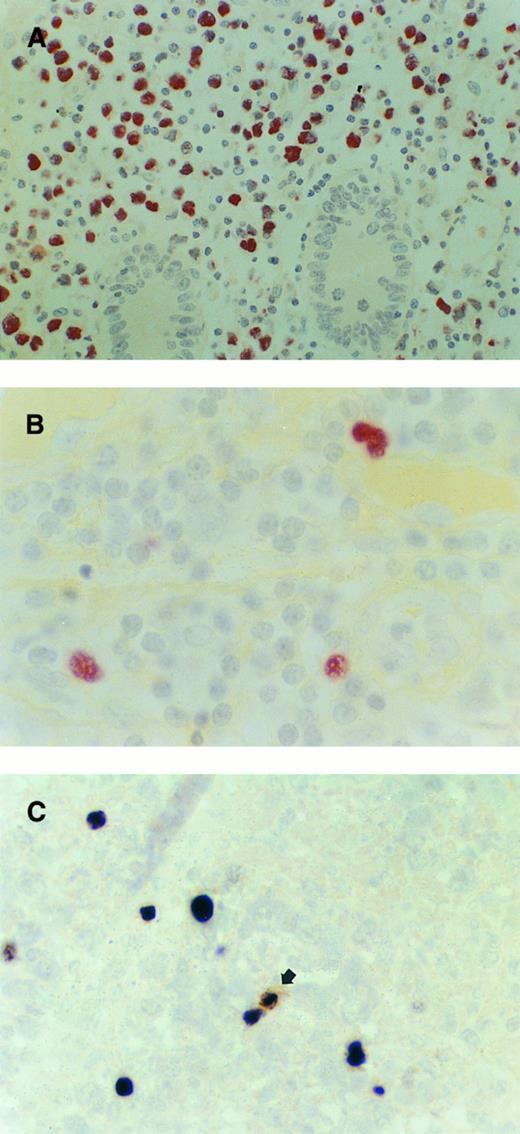
Intestinal T-NHL. (A) T-NHL, non-EATCL type. The vast majority of the neoplastic cells show strong expression of EBERs. Crypts are largely spared by the tumor cells. Case no. 1, original magnification ×400. (B) T-NHL, EATCL-like. Occasional large cells clearly identifiable as neoplastic show strong EBER expression. Case no. 7, original magnification ×1000. (C) T-NHL, non-EATCL type. A single small bystander cell (arrow) shows coexpression of EBERs and CD20 (L26). Some neoplastic large T cells lacking CD20 expression are positive for EBERs. Double staining for L26 (brown) and EBERs (black). Case no. 8, original magnification ×1000.
Intestinal T-NHL. (A) T-NHL, non-EATCL type. The vast majority of the neoplastic cells show strong expression of EBERs. Crypts are largely spared by the tumor cells. Case no. 1, original magnification ×400. (B) T-NHL, EATCL-like. Occasional large cells clearly identifiable as neoplastic show strong EBER expression. Case no. 7, original magnification ×1000. (C) T-NHL, non-EATCL type. A single small bystander cell (arrow) shows coexpression of EBERs and CD20 (L26). Some neoplastic large T cells lacking CD20 expression are positive for EBERs. Double staining for L26 (brown) and EBERs (black). Case no. 8, original magnification ×1000.
In nine additional cases, only rare EBER-positive small lymphocytes interpreted as latently infected, nonneoplastic bystander cells, were identified. In most cases, the excellent preservation of morphology allowed a clear-cut discrimination between EBER-positive nonneoplastic bystander cells and tumor cells. The double stainings for B- and T-cell antigens and EBERs in the cases with a minority of tumor cells (cases no. 6 to 8 and 11 to 13) revealed that many of the cells harboring EBERs were negative for B- and T-cell markers. In cases no. 6 to 8 (T-cell lymphomas), 10% to 20% of the large atypical EBER-positive cells coexpressed CD45RO (UCHL-1). In addition, small nonneoplastic lymphocytes coexpressing CD20 (L26) and EBERs were identified in case no. 8 (Fig 2C). In case no. 11, a follicular lymphoma, some neoplastic follicles contained groups of EBER-positive large blasts most of which lacked CD20 expression (Fig 3). The coexpression of CD20 was found in 15% of the EBER-positive cells, whereas UCHL-1 coexpression was identified in rare nonneoplastic lymphocytes. Case no. 12 showed coexpression of CD20 in 40% of the EBER-positive cells, whereas in case no. 13, a MALT low-grade lymphoma with significant plasmacytic differentiation, EBER expression was confined to CD20 negative cells morphologically corresponding to plasma cells.
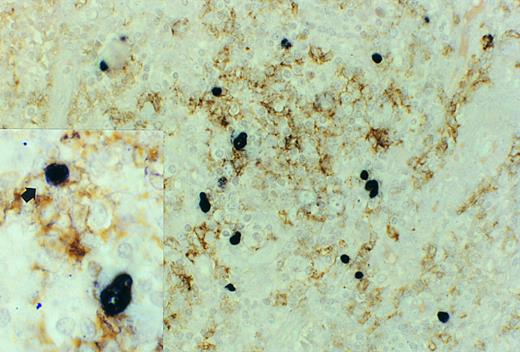
Follicle center cell lymphoma, double staining for CD20 (L26) and EBERs. The large, atypical EBER-positive blasts in the center of a neoplastic follicle do not express CD20. These cells were also negative for UCHL-1. Case no. 11, original magnification ×400. Inset, occasionally smaller cells (arrow) showed coexpression of EBERs and CD20. Original magnification ×1000.
Follicle center cell lymphoma, double staining for CD20 (L26) and EBERs. The large, atypical EBER-positive blasts in the center of a neoplastic follicle do not express CD20. These cells were also negative for UCHL-1. Case no. 11, original magnification ×400. Inset, occasionally smaller cells (arrow) showed coexpression of EBERs and CD20. Original magnification ×1000.
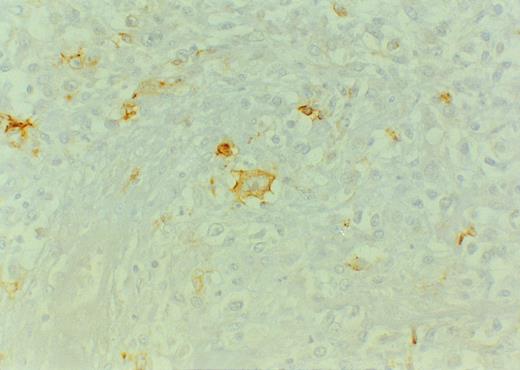
LMP-1 was positive only in 2 of 43 cases. The positive cells exhibited a typical membrane and perinuclear staining pattern and often resembled Hodgkin or Reed-Sternberg cells morphologically. One of the two cases, a sporadic high grade B-NHL from Mexico, showed discordance between LMP-1, which was consistently positive in a minority of neoplastic cells (Fig 4), and EBER ISH, which remained repeatedly negative, although a positive hybridization with the poly-d(T) probe confirmed the presence of intact mRNA. This case showed a lack of amplifiable DNA with the the β-globin gene primers; therefore, the presence of EBV could not be assessed by PCR.
Diffuse large B-cell lymphoma of Mexican origin, EBER-negative. Some large blasts reminiscent of Hodgkin cells strongly express LMP-1. Original magnification ×400.
Diffuse large B-cell lymphoma of Mexican origin, EBER-negative. Some large blasts reminiscent of Hodgkin cells strongly express LMP-1. Original magnification ×400.
CD30 was positive on paraffin sections in 11 of 43 cases (25.5%) including 2 of 27 sporadic B-NHL and 9 of 17 T-NHL. Four of the nine CD30-positive T-NHL were EBV-associated, whereas the remaining four EBV-associated T-NHL were CD30-negative; of these, two corresponded to the EATCL of Mexican origin, and two to pleomorphic medium and large cell T-NHL, one of Mexican and one of European origin. The two B-NHL which expressed CD30 corresponded morphologically to large cell (centroblastic) lymphomas, one of which was the LMP-1–positive and EBER–negative case referred to above.
The correlation between EBV PCR and EBER ISH was good. All EBER-positive lymphomas with amplifiable DNA (8 cases) were positive for EBV by PCR. In 6 cases positive for EBV by PCR, rare EBER-positive, nonneoplastic bystander cells were identified. However, five cases positive for EBV by PCR showed no EBER-positive cells in ISH, although the control mRNA hybridization confirmed the presence of intact mRNA. This probably implies the presence of EBV-infected bystander cells in the sections used for DNA extraction and their absence in the section examined by ISH. No EBER-positive cells were found in any of the examined cases negative for EBV by PCR. In eight cases unsuitable for PCR due to the absence of amplifiable DNA, the ISH study detected EBV-harboring cells (5 EBV-associated lymphomas and 3 cases with EBER-positive bystander cells).
DISCUSSION
The present study shows a high frequency of EBV-positivity in primary intestinal lymphoma of Mexican origin as compared with Western European cases. This difference is most striking in T-NHL, but also extends to a proportion of sporadic B-NHL.
In both B- and T-NHL a broad range of reactivity from 1% to 100% of the neoplastic population was found. Diffuse EBER-positivity (more than 50% of the tumor population) occurred in seven cases; in contrast, clusters of neoplastic cells (1% to 20% of the tumor population) occurred in six cases. We considered these two major patterns of EBER-positive tumor cells as EBV-associated NHL. However, in recent studies14,15,22 it has been suggested that the implications of these two patterns might be different. In the first group with diffuse positivity, it is likely that EBV infection occurred before or just after clonal expansion of tumor cells, and that the virus may have a central role in initiating and/or promoting lymphomagenesis. In the second group with foci of positive tumor cells, the role of the virus in lymphomagenesis is less understood. In these cases EBV infection might have occurred after clonal expansion. However an alternative explanation is that EBER negative tumor cells represent loss of the viral episome, which at some point in tumor progression no longer confers a proliferative or survival advantage to tumor cells.23
Recent studies in Western populations, as well as our findings in a Mexican population, indicate that T-NHLs are associated with EBV in a higher percentage than sporadic B-NHLs in nonimmunocompromised patients.8,9 As described by Hamilton-Dutoit and Pallesen, as well as others,8,9 the three sporadic B-NHL EBV-associated, other than Burkitt's type, showed presence of EBV in a minority of the identifiable tumor cells. However, in contrast to that study, none of our six cases with morphology similar to polymorphic large cell lymphoma were EBER-positive; this specific morphology was thought to be characteristic for one form of EBV-associated lymphoma. Interestingly, one of these cases showed discordant expression between LMP-1, which was consistently positive in a part of the large transformed immunoblasts occasionally resembling Reed-Sternberg cells, and EBER ISH, which remained repeatedly negative, in spite of a positive signal with the poly-d(T) probe which confirmed the presence of intact mRNA. We believe that a false negative reaction for EBERs seems improbable. There are two explanations for the absence of EBER expression in EBV-positive tumors. First, EBERs may not be expressed possibly due to down-regulation. In some cases of undifferentiated nasopharyngeal carcinoma, partial absence of EBER expression in cells that expressed LMP-1 has been shown.1,24 Second, the EBER genes may be deleted. Although this has never been reported to our knowledge, EBER gene deletions cannot be completely excluded, since recently, other deletions and rearrangements in the EBV genome mainly involving the BNLF-1 gene have been identified.25 This particular case, for purposes of analysis, was not included in the EBV-associated group due to the lack of signal in EBER ISH, despite the LMP positivity.
A special group among B-NHL is Burkitt's lymphoma. This group comprises two classic patterns: the endemic type in which almost 100% of the tumors are EBV-positive and the sporadic Western type in which the rate of EBV-positivity is low (5% to 30%).26 Our two cases, in concordance with recent series from other developing countries, suggest the existence of an intermediate group, which is similar in tumor localization and incidence to the sporadic Western type but has a higher frequency of EBV-positivity.26-28
The most striking finding in this study was the high prevalence of EBV in intestinal T-cell NHL in a Mexican population (100%), which is in contrast to what has been reported in the literature from Western Europe.8,13-15,22,29,30 Over the last few years, evidence has accumulated suggesting that the presence of EBV in extranodal T-cell lymphomas might be site-restricted.13-15 For instance, nasal T-cell lymphomas are EBV-associated neoplasms irrespective of the geographic location, whereas pulmonary and gastrointestinal T-cell lymphomas with or without angiocentricity are rarely associated with EBV.13,15 Our review of gastrointestinal T-cell NHL reported so far (Table 3), all of which have been in Western European populations, showed that among a total of 108 cases, 16 cases (14.8%) were EBV-associated.13,14,30-34 The majority of the analyzed cases (64 cases) correspond to the group of EATCL. The localization was mainly in the small intestine with few cases reported in the colon and stomach. Correlation of the presence of EBV with histologic type disclosed that non-EATCL are more frequently associated with EBV than EATCL (20.4% vs 10.9%, respectively). Because of these results, and in contrast to early observations,33 it was proposed in a recent editorial that EBV is not involved in the pathogenesis of EATCL.35 Although EATCL is rarely associated with EBV, it is interesting that the two Mexican cases that were analyzed were EBV-positive. The high prevalence of EBV-associated T-cell intestinal lymphoma in a Mexican population is certainly not a feature of immunosuppression, since none of the patients in our study were clinically immunocompromised. This finding is similar to what has been shown in Hodgkin's disease in Latin American countries, where the prevalence of EBV-associated neoplasms is higher than those in Western populations.36-39 One can speculate, as in HD, that the difference in EBV expression may reflect variations in the quality of the immune response mounted by patients from nutritionally and economically disadvantaged populations. An alternative explanation could be that this population is more exposed to gastrointestinal infections, and that this continuous exposure may render intraepithelial T cells of the intestine more vulnerable to EBV-infection due to excessive T-cell activation, probably at a younger age, increasing the risk for the development of EBV-associated lymphomas. Moreover, frequent presentation of EBV-positive T-NHL in nasopharyngeal lymphoepithelium, a site associated with productive viral replication, also suggests that T-cell infection is influenced by high viral load at selected sites.
LMP-1 expression was found only in 1 of 13 of the EBER-positive intestinal lymphomas. This discrepancy between the expression of EBER and LMP-1 has been previously recognized.9,13-16,23,29,31,40-45 The lack of expression of LMP-1 might be due to the insufficient sensitivity of the immunohistologic technique applied to wax-embedded tissue rather than frozen tissue sections.13 However, it is unlikely to be the only reason, as proper control tissues in the different studies yielded satisfactory staining. Furthermore, in two cases of T-NHL in our study, frozen tissue was available and LMP-1 was repeatedly negative. The lack of detection of LMP-1 in the presence of LMP-1 mRNA, found using reverse transcriptase-PCR, indicates that either the level of the protein is undetectable by the two monoclonal antibodies available or that LMP-1 mRNA is not always translated.29 In our series, the expression of both LMP and CD30 is less frequent than reported in the literature (Table 3). The expression of CD30 and LMP did not correlate with the amount of EBER-positive cells, because two cases with more than 80% of EBER-positive cells were CD30- and/or LMP-negative (Table 2).
The presence of EBV DNA was detected by PCR in 19 of 30 primary intestinal lymphomas (63%) in which amplifiable DNA could be extracted. However, in six of these cases, EBV was found by ISH exclusively in reactive small lymphocytes and rare blast cells, which were considered nonneoplastic bystander cells. In the double-staining technique many of the EBER-positive small lymphocytes that were regarded as latently infected B cells, as well as large atypical neoplastic cells in the EBV-positive tumors did not express T- nor B-cell markers. The frequent absence of surface marker expression has been noted previously both in reactive and neoplastic EBER-positive cells.9,13,46,47 The discrepancy in some cases between EBV-positivity in PCR, and absence of EBER-staining cells found in five of our cases is also a well known phenomenon,13 14 and most likely represents a sampling problem.
Another feature of interest in our study is the marked age difference between the European and the Mexican patients. Although the number of patients is small and is most probably influenced by demographic characteristics of the two populations and different patient selection criteria, other factors might be in part responsible for this difference. The highest differences in median age are present in the T-cell lymphoma (24 v 63 years) and the Burkitt's lymphoma groups (4 v 30 years), which show a universal association with EBV in the Mexican cases. This suggests that EBV might be a causing or promoting factor not only for Burkitt's lymphoma, but also for intestinal T-cell lymphoma, leading to an earlier manifestation of disease as compared with EBV-negative cases. Therefore, it seems that environmental or genetic factors might also be responsible for the younger average age of Mexican patients with intestinal T-cell lymphoma.
The numbers of T-NHL and Burkitt's lymphoma from Mexico is too small to allow any firm conclusion to be drawn. Nevertheless, our report is the first that describes that, similar to HD and probably Burkitt's lymphoma, there are important epidemiologic differences in EBV-association in intestinal NHL mainly of T-cell phenotype between Western European and Mexican populations. Whether these differences are the result of geography, ethnicity, socioeconomic factors, or other factors unrecognized as yet remains to be determined. More studies not only in intestinal NHL but also in nodal and other extranodal lymphomas from developing countries are needed to further confirm our data and clarify the reasons for this difference in the frequency of EBV infection.
ACKNOWLEDGMENT
We thank Bender Med Systems (Austria) for their financial contribution to the cost of the color reproductions.
Supported in part by the nominative chair, Silvia y Luis G. Aguilar.
Address reprint requests to Leticia Quintanilla-Martı́nez MD, Department of Pathology, University of Innsbruck. Müllerstr. 44, A-6020 Innsbruck, Austria.