Abstract
The present study was performed to investigate the early signalling events responsible for eosinophil activation in response to platelet-activating factor (PAF ), C5a, and leukotriene B4 (LTB4 ). We evaluated the effect of pertussis toxin (PTX) on eosinophil aggregation in vitro and cutaneous eosinophil recruitment in vivo. Further studies using the protein kinase inhibitors Ro 31-8220 and staurosporine were performed in vitro to assess in more detail the early signalling events induced by these three mediators. Our results show that C5a and LTB4 signal predominantly or exclusively through a PTX-sensitive G protein that is negatively modulated by protein kinase C, possibly at the level of phospholipase C-β. In contrast, PAF activates eosinophils independent of Gi by a mechanism that is abolished by Ro 31-8220, a selective protein kinase C inhibitor. In addition, these results show for the first time that a receptor-operated event on the eosinophil is essential for chemoattractant-induced eosinophil recruitment in vivo.
THE EOSINOPHIL IS thought to play an important role in the pathophysiology of parasitic and allergic diseases.1,2 In the former condition, eosinophil accumulation and activation at sites of migrating larvae may kill parasites or render them immobile.3 In addition, epidemiologic studies suggest a relationship between eosinophil levels in blood and protection against parasitic diseases.2 However, in allergic diseases, such as asthma, in which tissue eosinophilia is a common feature, uncontrolled eosinophil activation may lead to the release of lipid mediators, basic proteins, reactive oxygen species, and cytokines.4 These eosinophil products may cause bronchoconstriction, damage the respiratory epithelium, and contribute to airway hyperresponsiveness, a hallmark of asthma.5 It is thus possible that the development of pharmacologic agents that modulate eosinophil recruitment and activation in vivo may be of benefit in the treatment of allergic diseases.
We have recently shown that eosinophils, when activated with inflammatory mediators, including platelet-activating factor (PAF ), C5a, and leukotriene B4 (LTB4 ), undergo homotypic aggregation that is both time- and concentration-related and dependent on calcium and magnesium.6 Whereas the precise adhesion mechanisms responsible for eosinophil aggregation are not completely understood, we have shown the response to depend on CD18 and L-selectin, but not VLA-4, present on the eosinophil surface.7 The physiologic role of aggregation for eosinophil function in vivo is unclear, but this phenomenon has been observed after the intradermal injection of the chemokine RANTES in dog skin8 and it also occurs around migrating larvae of Schistosoma mansoni.3 It is possible that the close contact between these cells favors eosinophil priming, activation, and enhanced survival, via the release of granulocyte-macrophage colony-stimulating factor and interleukin-5 by eosinophils themselves.4 In addition, eosinophil aggregation may be an useful model to evaluate CD18- and L-selectin–dependent functional responses in vitro, as has been shown for neutrophils.9 10
The intracellular elevation of cyclic AMP is an effective means of modulating eosinophil activation in vitro and eosinophil recruitment in vivo.11,12 Recently, we have shown that the aggregation of eosinophils induced by PAF is significantly more sensitive to the inhibitory actions of cyclic AMP-elevating drugs, such as salbutamol and prostaglandin E1 , than equivalent responses evoked by C5a.13 The present study was performed to investigate the possibility that the early signalling events responsible for eosinophil homotypic aggregation and migration might differ depending on the nature of the activating stimulus, which could account for the difference in sensitivity to cyclic AMP. To this end, the effect of pertussis toxin (PTX) and inhibitors of protein kinase C (PKC) and cyclic AMP-dependent protein kinase (PKA) on eosinophil aggregation and, for comparison, Ca2+-mobilization was assessed for three agonists (PAF, C5a, and LTB4 ) that are known to elicit their effects by interacting with distinct G-protein–coupled receptors. Additional studies were also conducted to assess if PTX was able to distinguish between the effects of PAF, C5a, and LTB4 in an in vivo model of cutaneous eosinophilia.14-16 A preliminary account of some of these data was presented to the British Pharmacological Society.17
MATERIALS AND METHODS
Purification of guinea pig peritoneal eosinophils.The method of eosinophil purification is described in detail elsewhere.15 Briefly, ex-breeder female guinea pigs (Harlan, Oxon; 700 to 800 g) were treated with undiluted horse serum (1 mL intraperitoneally) every other day for 2 to 3 weeks and the cells were harvested by peritoneal lavage with heparinized saline (10 IU/mL) 2 days after the last injection. The cells obtained were layered onto a discontinuous Percoll-Hanks' balanced salt solution (HBSS; calcium- and magnesium-free) gradient followed by centrifugation (1,500g for 25 minutes at 20°C). Eosinophils (>95% pure, >98% viable) were collected from the 1.090/1.095 and 1.095/1.100 g/mL density interfaces. The cells were then washed twice in Dulbecco's phosphate-buffered saline (PBS; calcium- and magnesium-free, pH 7.4) to which glucose (10 mmol/L) was added.
Eosinophil aggregation.Aggregation experiments were performed as previously described.6 Briefly, guinea pig eosinophils were resuspended (5 × 106 cells/mL) in PBS, CaCl2 and MgCl2 (final concentrations, 1.0 mmol/L and 0.7 mmol/L, respectively) were added, and the cells were kept on ice. Five minutes before use, the cells were warmed to 37°C and aliquots (300 μL) were dispensed into siliconized cuvettes that were placed into a dual-channel platelet aggregometer (Chronolog 440 VS) linked to a dual pen recorder (Chronolog 707). The cells were incubated for at least 5 minutes at 37°C with continuous stirring at 700 rpm before stimulation with the indicated agonist. The reference cuvette contained buffer alone. Responses were allowed to develop for at least 3 minutes and were measured at the peak of aggregation. Results are expressed as the percentage of maximal aggregation induced by 10−7 mol/L phorbol myristate acetate (PMA). Eosinophils were incubated with the various pharmacologic agents (Ro 31-8220, staurosporine, and H89) for 2 to 3 minutes and then stimulated with PAF (10−10 mol/L to 10−7 mol/L), C5a (10−8 mol/L to 10−7 mol/L), or LTB4 (10−10 mol/L to 10−7 mol/L).
Measurement of intracellular calcium.Purified guinea pig eosinophils (5 × 106 cells/mL in PBS with 0.25% bovine serum albumin [BSA]) were loaded with fura-2-acetoxymethyl ester (2.5 μmol/L, 30 minutes at 37°C). After two washes, eosinophils were resuspended at 106 cells/mL in PBS buffer containing 10 mmol/L HEPES, 0.25% BSA, and 1 mmol/L calcium and stored on ice. Ten minutes before their use, eosinophils were warmed to 37°C and 300-μL aliquots were dispensed into quartz cuvettes. Changes in fluorescence were monitored at 37°C using a spectrometer (LS50; Perkin-Elmer Corp, Beaconsfield, Bucks, UK) at excitation wavelengths 340 nm and 380 nm and emission wavelength 510 nm. [Ca2+]i levels were calculated using the ratio of the two fluorescence readings and a kd for Ca2+ binding at 37°C of 224 nmol/L.18 As with the aggregation experiments, eosinophils were incubated with Ro 31-8220 and staurosporine for 3 minutes and then activated with PAF, C5a, or LTB4.
Radiolabeling of eosinophils and recruitment in vivo.Purified eosinophils were radiolabeled with 111In as previously described.14,16 The 111In-radiolabeled cells were then incubated at 37°C with PTX or vehicle for 2.5 hours (see below), washed twice, and injected intravenously (2.5 × 106 cells/animal) together with 125I-human serum albumin (125I-HSA; 5 μCi) into recipient guinea pigs (350 to 400 g) sedated with Hypnorm (0.15 mL intramuscularly). After 5 minutes, duplicate intradermal injections of inflammatory stimuli (PAF at 10−12 to 10−9 mol/site, LTB4 at 10−12 to 10−10 mol/site, and C5a at 10−12 and 10−11 mol/site) were administered in 0.1-mL volumes into the shaved dorsal skin following a randomized injection plan. 111In-labeled eosinophil accumulation and 125I-HSA extravasation were assessed 1 hour after intradermal injection of mediators. At this time, a blood sample was obtained by cardiac puncture, the animals were killed with an overdose of sodium pentobarbitone, the dorsal skin was removed and cleaned free of excess blood, and the sites were punched out with a 17-mm punch. The samples were counted in an automatic 5-head gamma-counter (Canberra Packard Ltd, Pangbourne, Berks, UK) and the counts were cross-channel corrected for the two isotopes. Eosinophil numbers in the skin sites were expressed as the number of 111In-eosinophils per skin site and 125I-HSA extravasation was expressed as microliters of plasma.14 16
PTX treatment.Purified eosinophils were diluted in PBS (5 × 106 cells/mL in PBS with 0.25% BSA and 10 mmol/L HEPES) and incubated with PTX (1 μg/mL) at 37°C for 2.5 hours. An aliquot was separated and labeled with Fura-2 as described above and the cells were incubated for a further 30 minutes. The eosinophils were washed and diluted in PBS containing 0.25% BSA and 10 mmol/L HEPES (5 × 106 cells/mL for the aggregation experiments and 106 cells/mL for calcium measurements). Magnesium (0.8 mmol/L, for the aggregation experiments only) and calcium (1.0 mmol/L) were added back and the cells were kept on ice until use. The activation of eosinophils with PAF, C5a, and LTB4 was performed on the same PTX-treated cell preparations. For the in vivo experiments, 111In-labeled eosinophils were diluted in PBS (5 × 106 cells/mL in PBS with 0.25% BSA and 10 mmol/L HEPES) and incubated with PTX (1 μg/mL) at 37°C for 2.5 hours. The cells were then washed twice and resuspended in a final volume of 5 × 106111In-eosinophils/mL.
Material.The following reagents were purchased from Sigma Chemical Co (Poole, UK): BSA, D-glucose, dimethyl sulphoxide (DMSO), PMA, PTX, and staurosporine. Horse serum, Dulbecco's PBS (calcium- and magnesium-free, pH 7.4), and HBSS were from Life Technologies Ltd (Paisley, UK). Percoll was from Pharmacia (Milton Keynes, UK). C16 PAF was from Bachem (Saffron Walden, UK) and LTB4 was from Cascade (Reading, UK). Human recombinant C5a (C5a) was a gift from Dr J. van Oostrum (Ciba Geigy, Summit, NJ). The protein kinase A inhibitor, H89, was purchased from Biomol (Nottingham, UK) and was dissolved in 50% ethanol. The protein kinase C inhibitor Ro 31-8220 was a gift from Dr D. Bradshaw (Roche, Welwyn Garden City, UK). Staurosporine and Ro 31-8220 were dissolved initially in DMSO and further diluted in appropriate assay buffer. None of the drug vehicles had any significant effect on aggregation or calcium responses induced by any of the agonists tested (data not shown).
Statistical analysis.Results were analyzed using analysis of variance (ANOVA) with the statistical program Instat (GraphPad Software V2.03). P values were assigned using Student-Newman-Keuls post-test. When only two groups were compared, the Student's t-test was performed. Results were considered significant when P < .05. Data are presented as the mean ± SEM of n experiments.
RESULTS
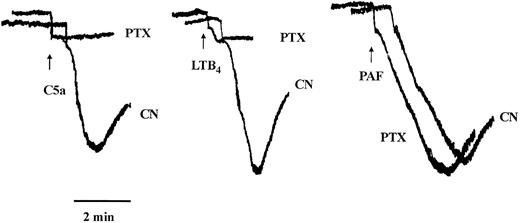
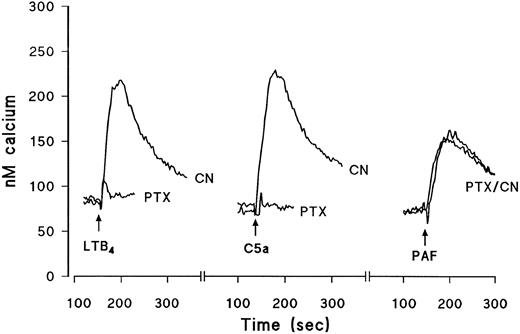
Effects of PTX on eosinophil aggregation and intracellular calcium transients.Experiments were designed to assess the nature of the G-proteins that couple to the seven domain transmembrane spanning receptors for PAF, C5a, and LTB4 by using the bacterial toxin, PTX. Eosinophils were pretreated for 2.5 hours with PTX (1 μg/mL) or its vehicle and agonist-induced aggregation and Ca2+ mobilization were subsequently assessed. PAF, C5a, and LTB4 elicited the aggregation of guinea pig eosinophils in a concentration-dependent manner with approximate EC50 values of 2 × 10−9 mol/L, 2 × 10−8 mol/L, and 3 × 10−9 mol/L, respectively (see Fig 4). Figures 1 and 2 show typical agonist-induced aggregation and intracellular calcium transient traces of control and PTX-treated cells. Whereas PAF-induced aggregation and Ca2+-mobilization were unaffected in PTX-treated eosinophils, the same responses evoked by LTB4 were markedly reduced (75% to 90%) and those effected by C5a were abolished (Table 1).
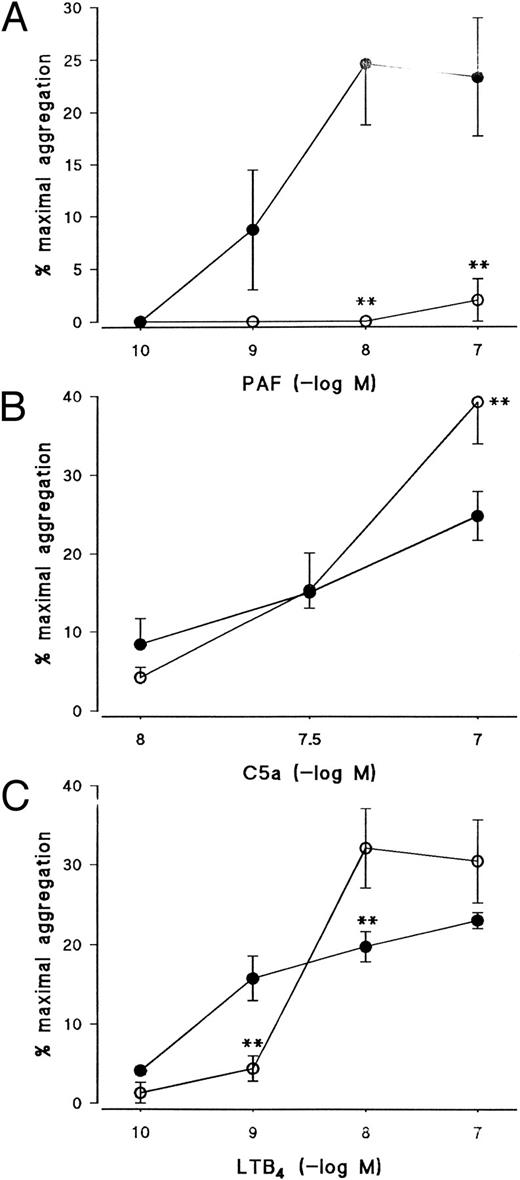
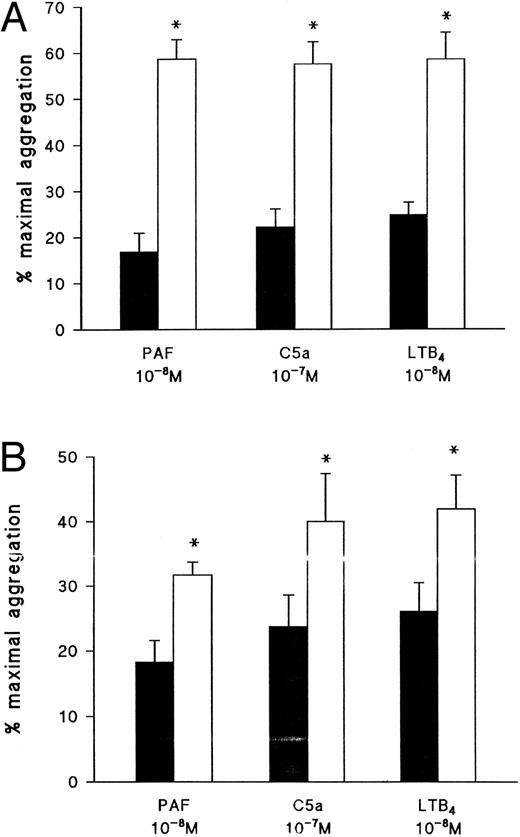
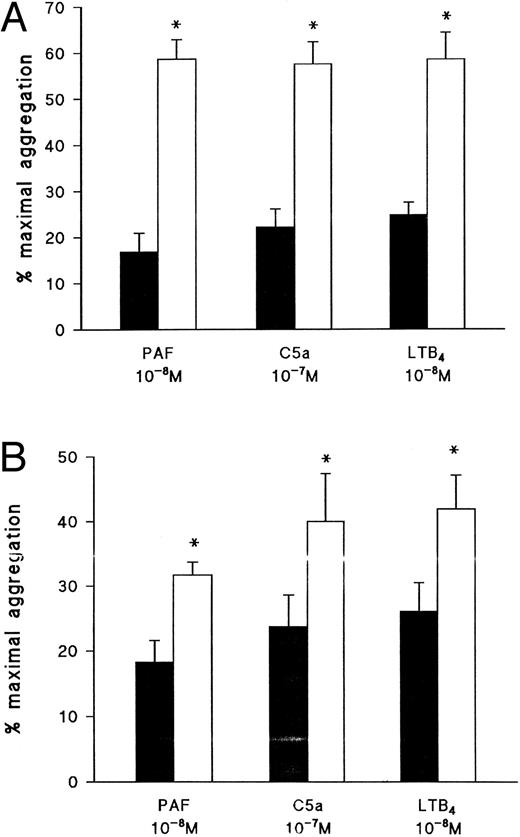
Effect of the PKC inhibitor, Ro 31-8220, on eosinophil aggregation induced by (A) PAF, (B) C5a, or (C) LTB4. Eosinophils were pretreated for 3 minutes with Ro 31-8220 (3 × 10−5 mol/L, open symbols) or vehicle (solid symbols) before the addition of PAF (10−10 mol/L to 10−7 mol/L), C5a (10−8 mol/L or 10−7 mol/L), or LTB4 (10−10 mol/L to 10−7 mol/L). Results are expressed as the percentage of maximal aggregation induced by PMA (10−7 mol/L) and each point is the mean ± SEM for three to five experiments. **P < .01 when compared with control values.
Effect of the PKC inhibitor, Ro 31-8220, on eosinophil aggregation induced by (A) PAF, (B) C5a, or (C) LTB4. Eosinophils were pretreated for 3 minutes with Ro 31-8220 (3 × 10−5 mol/L, open symbols) or vehicle (solid symbols) before the addition of PAF (10−10 mol/L to 10−7 mol/L), C5a (10−8 mol/L or 10−7 mol/L), or LTB4 (10−10 mol/L to 10−7 mol/L). Results are expressed as the percentage of maximal aggregation induced by PMA (10−7 mol/L) and each point is the mean ± SEM for three to five experiments. **P < .01 when compared with control values.
Typical traces showing the effect of PTX on eosinophil aggregation induced by maximally effective concentrations of C5a, LTB4 , or PAF. Eosinophils were pretreated for 2.5 hours with vehicle (CN) or PTX (1 μg/mL, see the Materials and Methods). The cells were washed, aliquots (300 μL) were placed in a cuvette, and changes in light transmission were recorded after activation with C5a (10−7 mol/L), LTB4 (10−8 mol/L), or PAF (10−8 mol/L). The time of the addition of the agonists is shown by the arrows. The initial rapid decrease in absorbance observed in all cases is an artifact due to the addition of the agonists.
Typical traces showing the effect of PTX on eosinophil aggregation induced by maximally effective concentrations of C5a, LTB4 , or PAF. Eosinophils were pretreated for 2.5 hours with vehicle (CN) or PTX (1 μg/mL, see the Materials and Methods). The cells were washed, aliquots (300 μL) were placed in a cuvette, and changes in light transmission were recorded after activation with C5a (10−7 mol/L), LTB4 (10−8 mol/L), or PAF (10−8 mol/L). The time of the addition of the agonists is shown by the arrows. The initial rapid decrease in absorbance observed in all cases is an artifact due to the addition of the agonists.
Typical traces showing the effect of PTX on intracellular Ca2+ transients induced by maximally effective concentrations of C5a, LTB4 , or PAF. Eosinophils were pretreated for 2.5 hours with vehicle (CN) or PTX (1 μg/mL, see the Materials and Methods). The cells were then labeled with fura-2-acetoxymethyl ester and washed and changes in fluorescence were monitored after activation with LTB4 (10−8 mol/L), C5a (10−7 mol/L), or PAF (10−8 mol/L). The time of the addition of the agonists is shown by the arrows. Results are expressed as the increase in the concentration of intracellular calcium (in nanomoles per liter) as a function of time (in seconds).
Typical traces showing the effect of PTX on intracellular Ca2+ transients induced by maximally effective concentrations of C5a, LTB4 , or PAF. Eosinophils were pretreated for 2.5 hours with vehicle (CN) or PTX (1 μg/mL, see the Materials and Methods). The cells were then labeled with fura-2-acetoxymethyl ester and washed and changes in fluorescence were monitored after activation with LTB4 (10−8 mol/L), C5a (10−7 mol/L), or PAF (10−8 mol/L). The time of the addition of the agonists is shown by the arrows. Results are expressed as the increase in the concentration of intracellular calcium (in nanomoles per liter) as a function of time (in seconds).
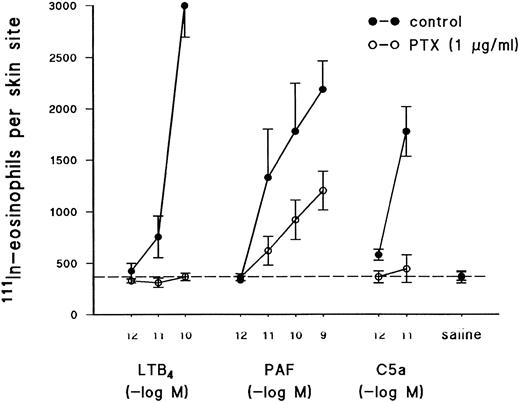
Effects of PTX on the recruitment of 111In-eosinophil into guinea pig skin.In agreement with previous reports,14,15 intradermal injection of LTB4 (10−12 to 10−10 mol/site), PAF (10−12 to 10−9 mol/site), and C5a (10−12 and 10−11 mol/site) into the skin of guinea pigs induced a dose-dependent recruitment of 111In-labeled eosinophils (Fig 3). Because the activation of seven transmembrane spanning G-protein–coupled receptors by inflammatory stimuli is believed to play a fundamental role in the recruitment of leukocytes to sites of inflammation in vivo,19 studies were performed to determine if the mechanism by which LTB4 , PAF, and C5a promote cutaneous eosinophilia and edema formation could be differentiated by PTX, as might be predicted from the in vitro results described above. As shown in Fig 3, the recruitment of 111In-eosinophils induced by C5a and LTB4 was abolished by pretreatment of eosinophils with PTX before their intravenous injection into recipient animals. In contrast, 111In-eosinophil accumulation induced by PAF was inhibited by approximately 50% in the same animals under identical experimental conditions (Fig 3). The reduction of the recruitment of 111In-eosinophils into skin sites could not be attributed to a reduction in the ability of PTX-treated cells to circulate in recipient animals. Indeed, the percentage of control 111In-eosinophils circulating at 1 hour was 10.8% ± 3.2% (n = 5) and the corresponding value for PTX-treated 111In-eosinophils was 15.3% ± 4.9% (n = 5).
Effect of PTX on the recruitment of 111In-eosinophils in response to intradermal injection of C5a, LTB4 , or PAF. Eosinophils were pretreated for 2.5 hours with PTX (1 μg/mL, see the Materials and Methods) or vehicle. The cells were then labeled with 111In and injected intravenously (2.5 × 106 cells/animal) into recipient guinea pigs. 111In-eosinophil recruitment was measured 1 hour after the intrademal injection of PAF (10−12 to 10−10 mol/site), C5a (10−12 and 10−11 mol/site), and LTB4 (10−12 to 10−10 mol/site). The dashed line across the graph represents background values in response to injection of saline. Results are the mean ± SEM of five pairs of animals.
Effect of PTX on the recruitment of 111In-eosinophils in response to intradermal injection of C5a, LTB4 , or PAF. Eosinophils were pretreated for 2.5 hours with PTX (1 μg/mL, see the Materials and Methods) or vehicle. The cells were then labeled with 111In and injected intravenously (2.5 × 106 cells/animal) into recipient guinea pigs. 111In-eosinophil recruitment was measured 1 hour after the intrademal injection of PAF (10−12 to 10−10 mol/site), C5a (10−12 and 10−11 mol/site), and LTB4 (10−12 to 10−10 mol/site). The dashed line across the graph represents background values in response to injection of saline. Results are the mean ± SEM of five pairs of animals.
The extravasation of 125I-HSA in response to PAF, C5a, and LTB4 was also measured in the same sites as 111In-eosinophil accumulation to confirm that animals that received PTX-treated cells responded normally to the intradermal stimuli. LTB4 induced no significant edema formation over saline background (data not shown), whereas PAF- and C5a-induced edema responses were unaltered in animals that received PTX- or vehicle-treated 111In-eosinophils (eg, control animals, PAF at 10−10 mol/site, 61.7 ± 6.8 μL of plasma, and C5a at 10−11 mol/site, 51.1 ± 6.7 μL; animals receiving PTX-treated eosinophils, PAF, 61.8 ± 5.2 μL, and C5a, 54.5 ± 3.0 μL; n = 5).
Effect of the protein kinase inhibitors Ro 31-8220, staurosporine, and H89 on eosinophil aggregation.Given that the results obtained with PTX indicate that the receptors for PAF, C5a, and LTB4 couple to distinct G-proteins in eosinophil membranes, further experiments were conducted to assess in more detail the early signalling events by which these agonists promote aggregation and Ca2+ mobilization. Pretreatment of eosinophils with the PKC inhibitor, Ro 31-8220, at a concentration (3 × 10−6 mol/L) that abolished PMA-induced aggregation (data not shown) suppressed PAF-induced aggregation by between 90% and 100% depending on the agonist concentration used (Fig 4A) but had little effect on aggregation evoked by C5a except at the highest concentration examined, in which aggregation was potentiated (Fig 4B). Curiously, aggregation induced by 10−10 and 10−9 mol/L LTB4 was markedly inhibited by Ro 31-8220 but was significantly enhanced when higher concentrations of the agonist were used (Fig 4C).
In contrast to the effects seen with Ro-31-8220, pretreatment of eosinophils with the nonspecific protein kinase inhibitor, staurosporine (10−7 mol/L), which inhibited PMA-induced aggregation by 76.6% ± 2.9% (n = 5), significantly augmented the same response evoked by PAF, C5a, and LTB4 (Fig 5A). Because staurosporine is known to inhibit protein kinases in addition to PKC,20 it was hypothesised that the augmentation of the aggregation response might be due to an effect on PKA that generally exerts an inhibitory effect in eosinophils (eg, Teixeira et al13 ). As shown in Fig 5B, the PKA inhibitor, H89, at a concentration (10−5 mol/L) shown previously to inhibit PKA in intact neutrophils,21 significantly enhanced PAF-, C5a-, and LTB4-induced eosinophil aggregation.
Effect of (A) staurosporine and (B) the protein kinase A inhibitor H89 on eosinophil aggregation induced by PAF, C5a, or LTB4 . Eosinophils were pretreated for 3 minutes with vehicle (▪), staurosporine (10−7 mol/L; □), or H89 (10−5 mol/L; □) before the addition of PAF (10−8 mol/L), C5a (10−7 mol/L), or LTB4 (10−8 mol/L). Results are expressed as the percentage of maximal aggregation induced by PMA (10−7 mol/L) and each point is the mean ± SEM for four experiments. *P < .05 when compared with control values.
Effect of (A) staurosporine and (B) the protein kinase A inhibitor H89 on eosinophil aggregation induced by PAF, C5a, or LTB4 . Eosinophils were pretreated for 3 minutes with vehicle (▪), staurosporine (10−7 mol/L; □), or H89 (10−5 mol/L; □) before the addition of PAF (10−8 mol/L), C5a (10−7 mol/L), or LTB4 (10−8 mol/L). Results are expressed as the percentage of maximal aggregation induced by PMA (10−7 mol/L) and each point is the mean ± SEM for four experiments. *P < .05 when compared with control values.
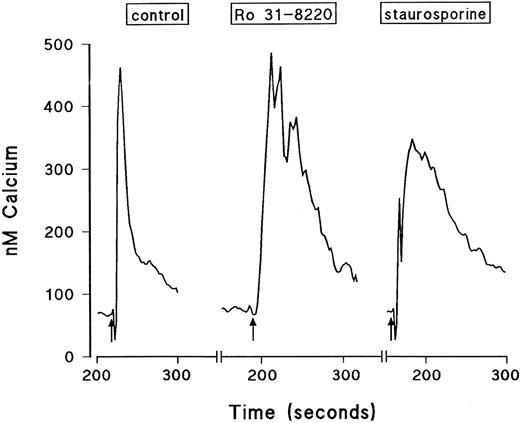
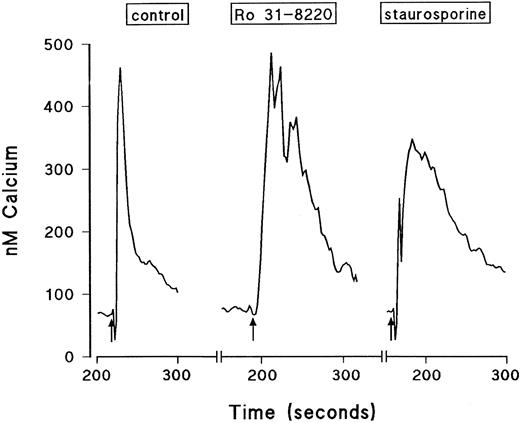
Effect of the protein kinase inhibitors Ro 31-8220 and staurosporine on Ca2+ mobilization in eosinophils.The effect of Ro 31-8220 and staurosporine on the Ca2+ transient evoked by PAF, C5a, and LTB4 is shown in Table 2. Data are expressed as the area under the curve, which takes into account both the amplitude and the duration of the Ca2+ signal. Whereas PAF-induced Ca2+ mobilization was abolished in eosinophils pretreated with Ro 31-8220 (3 × 10−5 mol/L), equivalent responses elicited by LTB4 and C5a were significantly enhanced. This effect was due predominantly to an increase in the duration rather than the peak height of the Ca2+ transient (see Fig 6 for a typical LTB4 response). Lower concentrations of C5a and LTB4 were affected similarly by pretreatment with Ro 31-8220 (data not shown). In contrast, pretreatment of eosinophils with staurosporine (10−7 mol/L) did not affect the Ca2+ transient evoked by PAF, whereas the same response elicited by C5a and LTB4 was markedly enhanced. Again, this effect was due predominantly to an increase in the duration rather than the amplitude of the Ca2+ transient (see Fig 6 for a typical LTB4 response).
Typical traces showing the effect of Ro 31-8220 and staurosporine on intracellular calcium transients induced by a maximally effective concentration of LTB4. Eosinophils were labeled with fura-2-acetoxymethyl ester and washed and changes in fluorescence were monitored. The cells were pretreated for 3 minutes with vehicle, Ro 31-8220 (3 × 10−5 mol/L), or staurosporine (10−7 mol/L) before the addition of LTB4 (10−8 mol/L, shown by the arrows). Results are expressed as the increase in intracellular calcium in nanomoles per liter as a function of time (in seconds).
Typical traces showing the effect of Ro 31-8220 and staurosporine on intracellular calcium transients induced by a maximally effective concentration of LTB4. Eosinophils were labeled with fura-2-acetoxymethyl ester and washed and changes in fluorescence were monitored. The cells were pretreated for 3 minutes with vehicle, Ro 31-8220 (3 × 10−5 mol/L), or staurosporine (10−7 mol/L) before the addition of LTB4 (10−8 mol/L, shown by the arrows). Results are expressed as the increase in intracellular calcium in nanomoles per liter as a function of time (in seconds).
DISCUSSION
In the present study the effects of PTX and inhibitors of PKC and PKA on PAF-, C5a-, and LTB4-induced homotypic aggregation and changes in the cytosolic free Ca2+ concentration in guinea pig eosinophils were assessed. Collectively, the results presented herein strongly suggest that the early signalling events that ultimately promote aggregation and Ca2+ mobilization differ fundamentally between the three stimuli. Unequivocal evidence for this contention was obtained from studies in which eosinophils were pretreated with PTX. Thus, whereas C5a-induced aggregation and Ca2+-mobilization were abolished in PTX-treated cells, the same responses evoked by LTB4 were inhibited by between 75% and 90% and those evoked by PAF were unaffected. The results with C5a are similar to those reported by Thelen et al,22 who showed that C5a-induced Ca2+ mobilization and degranulation of human eosinophils were abolished by PTX. Similarly, activation of the NADPH oxidase by LTB4 in guinea pig eosinophils and the elaboration of arachidonic acid were markedly inhibited by PTX.23 Collectively, these data clearly indicate that agonism of C5a and, in large part, LTB4 receptors on guinea pig eosinophils involves the activation of one or more PTX-sensitive, heterotrimeric G-proteins. Recent studies have established that guinea pig and human eosinophils express a number of G-proteins that include Gαi3 , Gαo , Gαq11 , and Gαs.24,25 Although it cannot be stated with certainty the type of G-protein involved in LTB4- and C5a-induced aggregation and Ca2+-mobilization, Gαo is unlikely to represent the PTX substrate because it is believed to be coupled primarily, although not exclusively (see Padrell et al26 ), to the activation of voltage-sensitive Ca2+ channels.27 Because Gαi1 is not present in guinea pig eosinophils25 a case can be made for Gαi2/Gαi3. In particular, both of these G-proteins are PTX substrates and couple to phospholipase (PLC)-β in a number of cells and tissues, which almost certainly include eosinophils (see below). The identity of the G-protein that promotes aggregation and Ca2+-mobilization in response to PAF is less clear. Although PTX failed to inhibit PAF-induced functional responses in eosinophils, the involvement of Gi was not initially excluded. It was reasoned that the PTX insensitivity might occur if Gi is predominantly precoupled to the PAF receptor, which would obscure the cysteine residue at the extreme carboxyl terminus of the protein and so reduce the efficiency of ADP-ribosylation. However, close inspection of the data suggests that, although this is a feasible explanation, it seems unlikely. Indeed, because the interaction of G-proteins with receptors is a dynamic process, logic dictates that PTX would have access to Gi , especially given the high concentration (1 μg/mL) used and the duration (2.5 hours) of exposure. Thus, if PAF receptors on eosinophils are coupled through Gi , an inhibitory effect on aggregation and Ca2+ mobilization would be expected. If Gi does not couple to PAF receptors, which other G-proteins could? Possible candidates include Gαq11 , which has been detected in guinea pig eosinophils,25 although the participation of Gαq16 , which is abundantly expressed in cells of the hematopoetic lineage,28,29 cannot be excluded. In contrast to our results in guinea pig eosinophils, the PAF receptor on human neutrophils is coupled to a PTX-sensitive G-protein.30 These findings suggest that the G-protein to which PAF receptors couple is species- and/or cell type-dependent, that multiple PAF receptors exist that signal through different G- proteins, or that PAF receptors can interact independently with multiple G-proteins.
There is much evidence to suggest that a receptor-operated event on neutrophils is important in mediating neutrophil recruitment in vivo.19,31 Indeed, the activation of leukocytes by inflammatory mediators appears to be essential for upregulation of integrins on their surface and subsequent firm adhesion to endothelial cells.19 As expected, in vitro pretreatment of eosinophils with PTX abolished 111In-eosinophil accumulation at skin sites induced by C5a and LTB4. In contrast, 111In-eosinophil accumulation induced by PAF was only inhibited by approximately 50% under identical experimental conditions. It is noteworthy that, in the same animals, edema responses induced by PAF and C5a were unaltered, showing that the animals that received PTX-treated eosinophils were responding normally to the exogenous intradermal stimulants. Collectively, our results provide convincing evidence that a receptor-operated event on the eosinophil is essential for eosinophil migration in vivo. These data are entirely consistent with what has been shown previously for neutrophils and lymphocytes.19,31 Furthermore, our in vivo results suggest that the early signalling events that ultimately induce 111In-eosinophil accumulation in guinea pig skin also differ between PAF, C5a, and LTB4. The partial inhibition of PAF-induced 111In-eosinophil accumulation by PTX was contrary to what would be predicted from the in vitro results. However, PAF-induced 111In-eosinophil accumulation in guinea pig skin is partially dependent on the release of a 5-lipoxygenase product, probably LTB4 ,16 which would account for the PTX-inhibitable component of PAF-induced cutaneous eosinophilia.
Further evidence for a difference in signalling between PAF, C5a, and LTB4 was obtained from the finding that PAF-induced eosinophil aggregation and Ca2+ mobilization were abolished by the PKC inhibitor, Ro 31-8220, whereas the duration of the Ca2+ transient evoked by C5a and LTB4 was prolonged, and eosinophil homotypic aggregation was either unaffected, inhibited, or enhanced (depending on the concentration of stimulus used; see Table 3). Thus, it is apparent from our data that PKC is an essential early signalling event after PAF activation of guinea pig eosinophil aggregation and Ca2+ mobilization. The results with C5a and LTB4 are consistent with data recently published by Perkins et al,32 who reported that Ro 31-8220 potentiated LTB4-induced hydrogen peroxide generation, the duration of the Ca2+ transient, and the magnitude of the inositol (1,4,5)trisphosphate [Ins(1,4,5)P3] signal in guinea pig eosinophils. Taken together, these data may be explained if PKC, when activated in response to C5a and LTB4 , exerts a negative feedback influence on one or more effectors of Ca2+ mobilization and homotypic aggregation that is relieved by Ro 31-8220. Although there are several proteins that could fulfill this role, including Ins(1,4,5)P3 5-phosphatase,33,34 the most likely target for PKC in this respect is PLC-β.35 Indeed, phosphorylation of PLC-β is regarded as a ubiquitous mechanism of regulation that results in a decrease in phosphoinositide hydrolysis and coincident desensitization of PLC-β–coupled receptors.35
The observation that the aforementioned effects of Ro 31-8220 were dependent on the activating stimulus is both intriguing and perplexing. This is confounded further by the finding that PAF and LTB4 promote the generation of Ins(1,4,5)P3 in guinea pig eosinophils,32,36 indicating that stimulation of PLC is an early signalling event that follows the ligation of both receptors. Based on available data, a number of possibilities could explain the divergent effects of Ro 31-8220 on agonist-induced Ca2+ mobilization and aggregation that are not mutually exclusive. First, PAF receptors may be coupled to an isoform of PLC that is not negatively regulated by PKC. Although the complement of PLC isoenzymes in eosinophils is unknown, a possible candidate could include PLC-ε, which has been identified as a major G-protein–regulated enzyme.37 A second explanation is based on the suggestion that phosphorylation of PLC-β by PKC decreases inositol phospholipid hydrolysis, probably by altering the efficiency by which it couples to the appropriate G-protein rather than by inhibiting enzyme activity per se.35 It is possible that phosporylation of PLC-β by PKC does not affect equally the coupling efficiency of all G-proteins.32,35 According to this paradigm, Giα2 (or Giα3 ) might not interact optimally with phosphorylated PLC-β under conditions in which the PTX-insensitive G-protein(s) that mediates the effects of PAF in eosinophils is not affected. Third, the potential multiplicity of βγ subunits liberated from activated G-proteins that are associated with PAF, LTB4 , and C5a receptors may exert opposing effects on the activity of PLC-β and/or other effectors of Ca2+-mobilization and aggregation. Finally, PKC may differentially mediate desensitization of PAF, LTB4 , and C5a receptors. However, this latter possibility is less likely, because activation of PKC with phorbol esters is accompanied by a diminished functional response of eosinophils to both PAF38 and LTB4.23
The inability of PTX to abolish LTB4-induced Ca2+ mobilization and aggregation suggests that LTB4 receptors might couple to more than a single G-protein. This contention is entirely consistent with the promiscuous coupling of other receptors to multiple G-proteins (eg, Asano et al39 and Rooney et al40 ) and is strengthened by the results obtained with Ro 31-8220, which both inhibited and potentiated aggregation depending on the concentration of LTB4 tested. It is noteworthy that previous studies have unequivocally identified two populations of LTB4 receptors on guinea pig eosinophils,41 42 which raises the possibility that they can independently couple to PTX-sensitive and -insensitive G-proteins. However, our results in vivo suggest that a PTX-sensitive pathway is most important for LTB4-induced eosinophil recruitment in guinea pig skin.
In contrast to the results obtained with Ro 31-8220, whose effects on eosinophil activation were agonist-dependent (see above), the nonselective protein kinase inhibitor, staurosporine,20 invariably augmented PAF-, C5a-, and LTB4-induced Ca2+-mobilization and aggregation. Although the mechanism of action of staurosporine was not formally investigated in this study, the finding that qualitatively identical data were obtained when H-89 was used suggests that staurosporine may be acting primarily as an inhibitor of PKA. Whereas Ro 31-8220 abolished PMA-induced aggregation, staurosporine blocked the responses by only 75%. Thus, it is possible that an effect of staurosporine on PKA may counteract the partial PKC inhibition and exert a negative tonic influence on Ca2+ homeostasis and the aggregation response in guinea pig eosinophils. Indeed, a similar conclusion was reported for PAF-induced platelet aggregation.43
In conclusion, the data presented herein suggest that the early signalling events that affect Ca2+-mobilization, homotypic aggregation, and in vivo recruitment of guinea pig eosinophils are dependent on the nature of the activating stimulus. Specifically, C5a and LTB4 signal predominantly or exclusively through a PTX-regulated pathway(s) that is negatively modulated by PKC, possibly at the level of PLC-β. In contrast, PAF activates eosinophils independent of Gi by a mechanism that is abolished by selective PKC inhibitors. Collectively, these results suggest that agonists can recruit multiple and parallel signalling pathways in guinea pig eosinophils that can ultimately evoke the same functional response. This observation provides a persuasive explanation for the difference in sensitivity of PAF-, C5a-, and LTB4-induced eosinophil responses to cyclic AMP-elevating agents reported in in vitro studies.13 Finally, these results showed for the first time that a receptor-operated event on the eosinophil is essential for PAF, C5a, and LTB4-induced eosinophil recruitment in vivo.
Supported by the National Asthma Campaign (UK), The Medical Research Council (UK), The Wellcome Trust, The British Lung Foundation (BLF ), and Sandoz (Switzerland).
Address reprint requests to Mauro M. Teixeira, MD, PhD, Applied Pharmacology, Imperial College of Medicine at the National Heart and Lung Institute, Dovehouse Street, London SW3 6LY, UK.