Abstract
The majority of BALB/c mice immunized with the BCL1 lymphoma-derived idiotype (Id+) IgM and subsequently challenged with BCL1 tumor cells develop a state of tumor dormancy. The vast majority of dormant lymphoma cells are in cell cycle arrest, but there are also residual replicating cells. In the present studies, we attempted to define features of both the dormant lymphoma cells and the host that lead to escape from dormancy. Escape from dormancy occurs at a steady rate over a 2-year period, suggesting that it is a stochastic process. We found that, in the majority of mice, escape was due to the emergence of genetic variants that were no longer susceptible to the anti-Id–mediated induction of dormancy. Ten percent of these variants were Id−; the remainder were Id+ but could grow in the presence of anti-Id antibodies, suggesting that there were mutations in molecules involved in one or more mIg-mediated negative-signaling pathways. In two of five such escapees, alterations in either Syk, HS1, and/or Lyn were observed. In a small percentage of mice, a low titer of circulating anti-Id antibody before tumor challenge correlated with a subsequent, more rapid loss of dormancy.
CANCER DORMANCY is a well-recognized clinical phenomenon in which tumor cells are present but the tumor burden does not increase for long periods of time.1-5 However, tumor cells can regrow many years or even decades later.
We have had a long-term interest in cancer dormancy emanating from studies of a murine lymphoma/leukemia (BCL1 ) in which we encountered dormancy early in our investigations with immunotoxin therapy.6 BCL1 was the first B-cell lymphoma described in mice.7 It arose spontaneously in an elderly BALB/c mouse and is characterized by early splenomegaly and later leukemia. Lymph nodes are not enlarged until late in the course of the disease.8 The tumor cells express IgMλ and IgDλ; the two isotypes share a common idiotype (Id) as defined serologically and by sequence analysis.8 9
To gain more insight into the mechanisms underlying tumor dormancy, we10-12 and others13,14 have studied BCL1 tumor dormancy in mice immunized with the BCL1 IgM. About 70% of immunized mice do not develop splenomegaly by 60 days11 and this time point (and later) defines dormancy. The dormant state lasts for many months (or years), but dormant tumors can regrow at a later time. The aim of this study was to understand events in both the host and the tumor cells responsible for the outgrowth of dormant lymphoma cells (DLCs) in Id-immunized mice.
MATERIALS AND METHODS
Animals and BCL1 tumors.BALB/c mice were obtained from our University Animal Resources Center (ARC). SCID mice were purchased from the University of Wisconsin and housed and maintained in modified barrier facilities by the ARC. The BCL1 tumor was maintained in vivo by intravenous and intraperitoneal passage in BALB/c mice. (We call these cells wild-type.) The cells bear μλ3 and δλ3 . Five weeks after passage of 5 to 10 × 105 tumor cells, mice were killed and their splenocytes were used as a source of tumor cells. The presence of BCL1 tumor cells in dormant mice was determined by injecting graded numbers of splenocytes from these mice into naive BALB/c mice and/or by FACS analysis of splenocytes.
The assessment of splenomegaly for monitoring tumor growth.To follow the growth of tumor cells over long periods of time, it was necessary to use a noninvasive, quantitative measurement of tumor growth. Previous studies have established that the spleen is the first and primary site of tumor growth in BALB/c mice.8 We, therefore, evaluated splenic enlargement by blinded palpation of a large number of animals to determine the suitability of this method for quantifying tumor.
Determination of splenomegaly by physical palpation.To assign values to the degree of splenomegaly, the ventral surface of the mouse was divided into four equal quadrants progressing from the left rib cage to the right rib cage. Each quadrant was assigned a splenic index with values of 1, 2, 3, and 4; the median line corresponds to the value of 2 (Fig 1). Spleens from normal 12-week-old BALB/c mice have a value of 0.5; ie, the spleen extends halfway between the left rib cage and the first quadrant line that is given the value of 1, a spleen that extends to the median line was given a value of 2, one that extends halfway between the median line and right rib cage was given a value of 3, and one that extends to the right rib cage has a value of 4. If the tip of the spleen is located between two of the primary quadrants, the distance between the lines is estimated in increments of one-eighths. Immunization with the BCL1 Id can result in an increase in spleen size to 1 and occasionally 1.5. Therefore, we selected a spleen index of ≥2 as indicative of tumor growth in BCL1 Id immunized animals. The reproducibility of the spleen palpation was shown by the close correlation of repeated blinded determinations by a single individual using the same animals and the close correlation of independent determinations of splenomegaly by separate individuals. All determinations of splenomegaly were performed in a blinded manner. As shown in Fig 2A, palpation is highly effective at quantifying BCL1 cells in the spleen. In addition, the time of onset of splenomegaly after transferring the BCL1 cells into naive recipients represents an additional means of quantifying the tumor burden (Fig 2B).
Determination of spleen indices. The ventral surface of the mouse was divided into four equal quadrants progressing from the left rib cage to the right rib cage. The terminus of each quadrant was assigned a splenic index of 1, 2 (median line of the mouse), 3, and 4 (right rib cage) (see solid lines in figure). Representations of spleens with splenic indices of 1 (black), 2 (dark gray), 3 (light gray), and 4 (white) bordered by black are illustrated. The costal margin is drawn to improve clarity.
Determination of spleen indices. The ventral surface of the mouse was divided into four equal quadrants progressing from the left rib cage to the right rib cage. The terminus of each quadrant was assigned a splenic index of 1, 2 (median line of the mouse), 3, and 4 (right rib cage) (see solid lines in figure). Representations of spleens with splenic indices of 1 (black), 2 (dark gray), 3 (light gray), and 4 (white) bordered by black are illustrated. The costal margin is drawn to improve clarity.
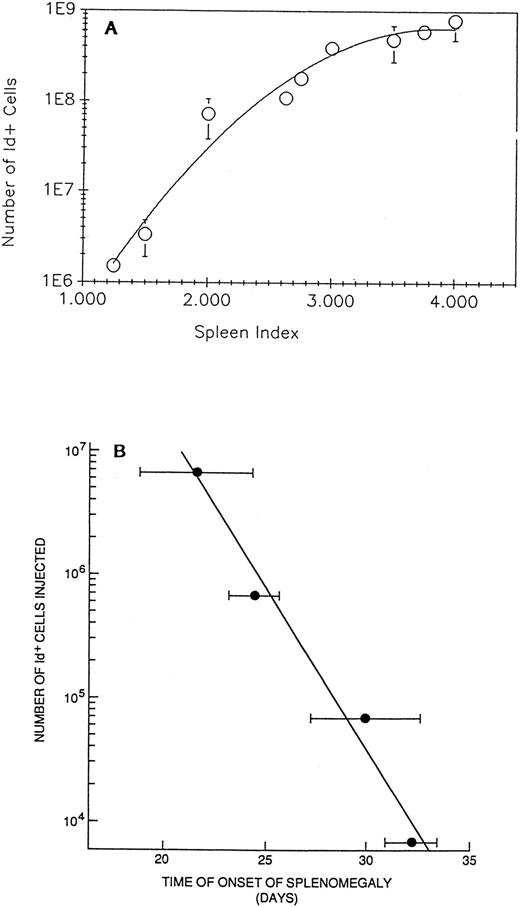
(A) Splenomegaly, as measured by palpation, is proportional to the number of BCL1 cells in the spleen. Naive BALB/c mice injected with 106 BCL1 cells were palpated and their spleen size was recorded single-blinded. The size was graded as 1 to 4 (spleen index), with ≥2 representing tumor growth (see the Materials and Methods). The animals were killed, the cells were stained, and the number of Id+ cells was determined by FACS analysis. (B) Time of onset of splenomegaly as determined by palpation correlates with the number of BCL1 cells previously injected. Groups of five animals each were injected with graded numbers of BCL1 cells as determined by Id analysis. Splenomegaly was determined by a single observer without knowledge of the treatment protocol. Bars are the SEM.
(A) Splenomegaly, as measured by palpation, is proportional to the number of BCL1 cells in the spleen. Naive BALB/c mice injected with 106 BCL1 cells were palpated and their spleen size was recorded single-blinded. The size was graded as 1 to 4 (spleen index), with ≥2 representing tumor growth (see the Materials and Methods). The animals were killed, the cells were stained, and the number of Id+ cells was determined by FACS analysis. (B) Time of onset of splenomegaly as determined by palpation correlates with the number of BCL1 cells previously injected. Groups of five animals each were injected with graded numbers of BCL1 cells as determined by Id analysis. Splenomegaly was determined by a single observer without knowledge of the treatment protocol. Bars are the SEM.
Antibodies.To prepare polyclonal anti-Id, the BCL1 Id was conjugated to keyhole limpet hemocyanin (KLH) and injected into BALB/c mice.11 The total levels of polyclonal mouse IgG anti-BCL1 Id in sera were determined by radioimmunoassay, as previously reported.11 Polyclonal mouse anti-BCL1 Id (MABCL1Id) ascites was produced by intraperitoneal injection of pristane into Id-immune mice followed 1 to 2 weeks later by an intraperitoneal injection of SP2/0 myeloma cells.15,16 6A5 hybridoma cells secreting rat IgG2a anti-BCL1 Id were obtained from Dr Freda Stevenson (Southampton, UK). The C5D5 BCL1 Id+ IgM λ was obtained by fusing BCL1 cells with SP2/0 myeloma cells and purifying the Id+ IgM. Monoclonal B1.1 rat antimouse λ3 (RtAMλ)17 was generated and characterized as described. Normal rat Ig (NRtIg) was purified from pooled rat serum by chromatography on diethyl aminoethyl-Sephadex A-50. Rabbit antimouse μ (RAMμ) and rabbit antimouse Ig (RAMIg) were prepared as described.18 Other antibodies included monoclonal rat antimouse IgM (PharMingen, San Diego, CA),11 mouse antirat λ-1 (PharMingen),12 rabbit anti-HS1 peptide (RbAHS1),19 and monoclonal anti-HS1 (MAHS1).20 21 Rabbit anti-Lyn (RbALyn) sera were produced by immunization with a GST-containing fusion protein incorporating a mouse-Lyn specific sequence located between positions 10 and 66. To prepare rabbit anti-Syk sera (RbASyk), rabbits were immunized with a high-performance liquid chromatography-purified synthetic peptide containing the 28-C-terminal amino acids of the human Syk protein bound to KLH in the presence of carbodimide.
Cytofluorometry.Stained DLCs or wild-type BCL1 cells were analyzed on a FACScan or FACStar Plus equipped with argon ion and helium neon lasers tuned at 488 and 633 nm, respectively (Becton Dickinson, San Jose, CA). Forward or orthogonal light scattering and 3 to 4 fluorochrome emission signals were recorded for each cell. Data were analyzed by Paint-a-Gate software as described.22 This analysis enabled us to perform a multidimensional identification of cells reactive with the antibodies as well as the determination of their relative size (small v large) based on the position of the cells in the correlative display of the forward versus the orthogonal light scattering. The number of BCL1 cells could be measured by determining either the number of Id+ cells or the number of large λ+3 cells in the tumor-bearing mice minus the number of large λ+3 cells in Id-immune non–tumor-bearing mice. The results were similar using these two methods.
Phenotyping.Cells (106) were incubated with either anti-λ or anti-Id antibodies. Binding was detected using a secondary fluorescein isothiocyanate (FITC) mouse antirat κ (MARK) antibody (Becton Dickinson). Alternatively, cells were incubated with a biotinylated anti-λ or anti-Id and incubated with FITC-antimouse IgM and the bound biotinylated antibody was detected with phycoerythrin-streptavidin. Similar results were obtained from staining with anti-Id or anti-λ if the small λ+ cells were excluded. The latter represents about 0.25% of nucleated cells per spleen. Cells were stained at 4°C for 15 minutes. After the final wash, cells were resuspended in 1% paraformaldehyde and maintained at 4°C in the dark before analysis. Control values were subtracted.
Determination of tumor growth in Id-immune mice.Id-immune and control mice were injected with 106 escapee tumor cells or 106 wild-type BCL1 tumor cells as a control and spleens were palpated twice weekly. The day of onset of splenomegaly was recorded for each animal and experimental values were compared with controls by t-test to determine if they were different.11 12
Immunoblotting.Cells (1 × 107) were washed once in phosphate-buffered saline before lysis in 100 μL lysis buffer for 20 minutes on a shaker at 4°C. After removal of insoluble material at 13,000 RPM for 10 minutes, an equal volume of 2× sodium dodecyl sulfate (SDS) Laemmli sample buffer23 was added to the cell lysate. Protein transfer and immunodetection were performed as described.24 For detection of phosphotyrosil residues, the primary monoclonal antibody (MoAb) was B-PY-20 and the secondary reagent was HRP-SA.
Immune complex kinase assay.Cells (5 × 106) were lysed in 0.5 mL lysis buffer and the kinases were immunoprecipitated by antibodies against Lyn or Syk. The beads were washed twice with kinase buffer (20 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 5 mmol/L MnCl2 , 5 mmol/L MgCl2 ) and resuspended in 50 μL of the same buffer before adding 1 μmol/L cold ATP and 10 μCi γ-[32P]ATP (Amersham, Arlington Heights, IL).25 After incubation for 15 minutes at room temperature, the beads were washed five times with washing buffer and the proteins were eluted in 70 μL Laemmli SDS sample buffer. Thirty-five microliters from each sample was electrophoresed on 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and the gel was fixed in 15% acetic acid for 30 minutes, washed, and dried. The radioactive bands were visualized and analyzed using a PhosphoroImager System (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Growth of DLCs in Id-immune mice.The kinetics of tumor growth of BCL1 cells in Id-immune and naive BALB/c mice are shown in Fig 3. In about 70% of Id-immune mice, there was no detectable splenomegaly and no increase in BCL1 Id+ cells for 60 days or more.11 In contrast, an increase in both splenomegaly and Id+ cells in naive mice injected with BCL1 cells was detected by 1 month. Hence, 60 days was used as the operational definition of dormancy in these (as in previous) studies.
Progressive tumor growth in nonimmune versus Id-immune mice challenged with BCL1 tumor cells. (A) The spleens from naive (○) and Id-immune (•) BALB/c mice injected with 106 BCL1 cells were palpated as described11 on the days indicated. Naive mice have an average spleen size of ∼0.5. Immunization with BCL1 Id results in a spleen size of 1 to 1.5 before BCL1 tumor injection. (B) BCL1 cells (1 × 106) were injected into naive BALB/c mice (○) or into Id-immune BALB/c mice (•). At the times indicated, mice were killed and the number of BCL1 tumor cells was determined by staining with RtABCL1Id for naive animals or RtAMλ for Id-immune animals (endogenous mouse anti-BCL1 Id [MA BCL1 Id] blocks staining with RtABCL1-Id but not the RtAMλ Ab) and analyzed by FACS.
Progressive tumor growth in nonimmune versus Id-immune mice challenged with BCL1 tumor cells. (A) The spleens from naive (○) and Id-immune (•) BALB/c mice injected with 106 BCL1 cells were palpated as described11 on the days indicated. Naive mice have an average spleen size of ∼0.5. Immunization with BCL1 Id results in a spleen size of 1 to 1.5 before BCL1 tumor injection. (B) BCL1 cells (1 × 106) were injected into naive BALB/c mice (○) or into Id-immune BALB/c mice (•). At the times indicated, mice were killed and the number of BCL1 tumor cells was determined by staining with RtABCL1Id for naive animals or RtAMλ for Id-immune animals (endogenous mouse anti-BCL1 Id [MA BCL1 Id] blocks staining with RtABCL1-Id but not the RtAMλ Ab) and analyzed by FACS.
Size of the population of DLCs with time after tumor cell challenge.Lack of progressive splenomegaly could be caused by an absence of BCL1 cells in the animal, slow growth of tumor cells due to the ongoing specific immune response, or the presence of a population of tumor cells that is not growing or slowly declining in size. In an attempt to distinguish among these possibilities, dormant Id-immune mice (ie, without splenomegaly) were killed at various times up to 210 days after BCL1 challenge and cells from their spleens were analyzed by flow cytometry for the number of Id+ BCL1 cells. As shown in Fig 4, there was no significant change in the size of the DLC population during the time of observation. Three additional mice were analyzed as late as 450 days. They each had 0.5 to 1 × 106 DLCs in their spleens. These results exclude the explanations that dormancy is due to an absence of tumor cells or to the slow growth of tumor. Rather, a state of dormancy was caused by the failure of the existing DLC population to expand, ie, cells were dividing and dying at the same rate and/or were cell cycle-arrested.
The population of DLCs is stable in size. Id-immune mice carrying dormant tumors were killed at various times. The number of large λ+3 cells was determined by FACS analysis. The number in each column indicates the number of mice analyzed for that time frame with the standard deviation indicated by the error bars. The average number of λ+3 cells in Id-immune animals not injected with BCL1 cells (normal B-cell blasts, 0.24 ± 0.09; N = 6) was subtracted from the value obtained for each animal before each group was averaged. The percentage of dormant tumor is the percentage of corrected λ+3 cells in the total splenocyte population for each animal.
The population of DLCs is stable in size. Id-immune mice carrying dormant tumors were killed at various times. The number of large λ+3 cells was determined by FACS analysis. The number in each column indicates the number of mice analyzed for that time frame with the standard deviation indicated by the error bars. The average number of λ+3 cells in Id-immune animals not injected with BCL1 cells (normal B-cell blasts, 0.24 ± 0.09; N = 6) was subtracted from the value obtained for each animal before each group was averaged. The percentage of dormant tumor is the percentage of corrected λ+3 cells in the total splenocyte population for each animal.
Malignant potential of DLCs.The next question that arose was whether the DLCs had lost their malignant potential or whether they were fully malignant but maintained in a dormant state. To answer this question, normal mice were injected with various numbers of either DLCs from Id-immune mice or wild-type BCL1 cells from nonimmune mice. Preliminary experiments indicated that cell sorting after staining with anti-λ decreased the tumorigenicity of both wild-type BCL1 cells and the DLCs. Hence, in these experiments, aliquots of the donor tumor cell suspension were assayed for the number of tumor cells present; subsequently, separate unsorted, unstained cells were transferred. In addition, we determined whether cotransfer of B and T cells from Id-immune mice and exposure to anti-Id could influence the behavior of wild-type BCL1 cells in the naive recipient.
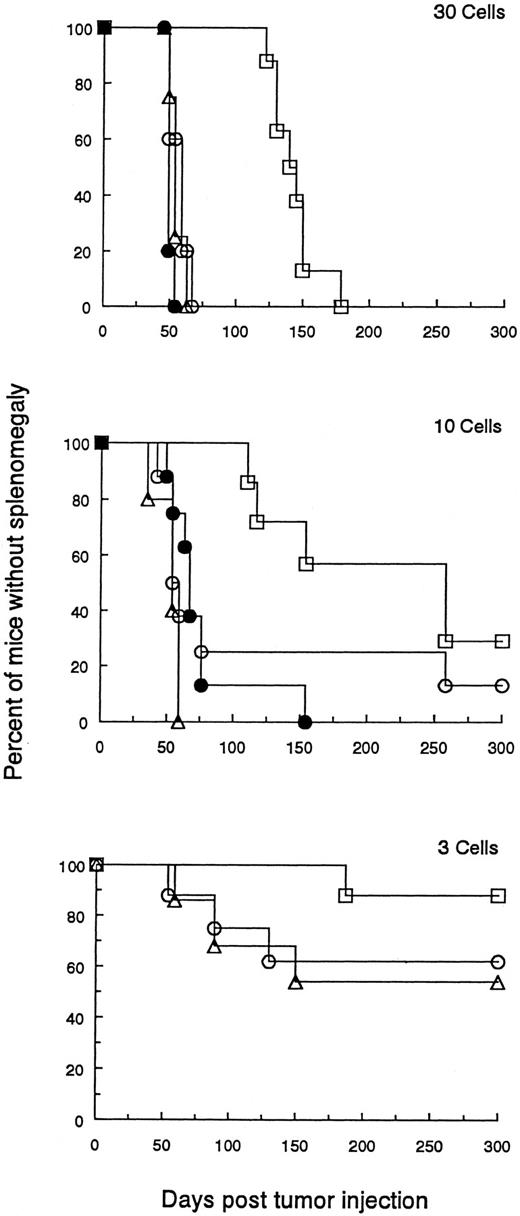
The malignant potential of DLCs. The percentage of tumor cells was determined by FACS analysis in a population of splenocytes from BCL1 wild-type or DLC-containing spleens. Naive animals were then injected with graded numbers of wild-type BCL1 tumor cells (▵), DLC tumor cells (□), or wild-type BCL1 tumor cells pulsed with 100 μg MαBCL1Id, and co-mixed with 106 splenocytes from BCL1 -Id–immunized mice (○) or wild-type BCL1 tumor cells admixed with a pool of 106 splenocytes from mice containing 0.72% DLCs (•). Cell numbers were 100 (not shown), 30, 10, 6 (not shown), 3, and 1 (not shown). A Breslow-Day test was used to examine the differences between BCL1 and DLC treatment groups across cell number groupings for the occurrence of splenomegaly. Comparisons of time to splenomegaly across treatment groups were provided by numbers of cells entered using a log-rank test (product-limit survival estimates enter into this computation). Multiple comparisons between pairs of treatments were accomplished using a Bonferonni correction to the P values for significant results. Mantel-Haenszel χ2 tests were used to provide an indication of the tendency to increase the occurrence of splenomegaly with increased cell numbers. In comparing BCL1 to DLCs for occurrence of splenomegaly, the Breslow-Day test comparing equal odds ratios of occurrence between the BCL1 and DLCs across cell numbers was not significant (P = .627). The overall odds ratio estimate is 6.103 for likelihood of splenomegaly from BCL1 compared with DLCs. The comparison by log-rank test for time to splenomegaly for mice receiving DLCs versus the other treatment groups was significantly different for cell numbers (P values in parentheses) of 100 (.0001), 30 (.0001), 10 (.0031), and 6 (.0194). No statistical difference was observed when 3 (.351) or 1 (.085) cells were injected. (Analogous results were also seen when the data were analyzed by a standard t-test.) There were no statistical differences in occurrence of splenomegaly or time to splenomegaly among the groups (BCL1 , treated BCL1 , or the BCL1 plus DLCs). Mantel-Haenszel statistics indicate an increased occurrence of splenomegaly for increased cell numbers (P values in parentheses): BCL1 (.001), DLC (.001), and treated BCL1 (.005).
The malignant potential of DLCs. The percentage of tumor cells was determined by FACS analysis in a population of splenocytes from BCL1 wild-type or DLC-containing spleens. Naive animals were then injected with graded numbers of wild-type BCL1 tumor cells (▵), DLC tumor cells (□), or wild-type BCL1 tumor cells pulsed with 100 μg MαBCL1Id, and co-mixed with 106 splenocytes from BCL1 -Id–immunized mice (○) or wild-type BCL1 tumor cells admixed with a pool of 106 splenocytes from mice containing 0.72% DLCs (•). Cell numbers were 100 (not shown), 30, 10, 6 (not shown), 3, and 1 (not shown). A Breslow-Day test was used to examine the differences between BCL1 and DLC treatment groups across cell number groupings for the occurrence of splenomegaly. Comparisons of time to splenomegaly across treatment groups were provided by numbers of cells entered using a log-rank test (product-limit survival estimates enter into this computation). Multiple comparisons between pairs of treatments were accomplished using a Bonferonni correction to the P values for significant results. Mantel-Haenszel χ2 tests were used to provide an indication of the tendency to increase the occurrence of splenomegaly with increased cell numbers. In comparing BCL1 to DLCs for occurrence of splenomegaly, the Breslow-Day test comparing equal odds ratios of occurrence between the BCL1 and DLCs across cell numbers was not significant (P = .627). The overall odds ratio estimate is 6.103 for likelihood of splenomegaly from BCL1 compared with DLCs. The comparison by log-rank test for time to splenomegaly for mice receiving DLCs versus the other treatment groups was significantly different for cell numbers (P values in parentheses) of 100 (.0001), 30 (.0001), 10 (.0031), and 6 (.0194). No statistical difference was observed when 3 (.351) or 1 (.085) cells were injected. (Analogous results were also seen when the data were analyzed by a standard t-test.) There were no statistical differences in occurrence of splenomegaly or time to splenomegaly among the groups (BCL1 , treated BCL1 , or the BCL1 plus DLCs). Mantel-Haenszel statistics indicate an increased occurrence of splenomegaly for increased cell numbers (P values in parentheses): BCL1 (.001), DLC (.001), and treated BCL1 (.005).
Figure 5 shows a representative experiment of two performed. There was no significant difference in the number of DLCs compared with wild-type BCL1 cells needed to adoptively transfer progressive tumor growth in recipients.
However, the results did show a significantly longer delay between the regrowth of DLCs as compared with the same number of wild-type BCL1 cells. This could not be accounted for by the transfer of Id-immune B or T cells from the donor spleen because the addition of 99% Id-immune spleen cells from dormant mice to wild-type BCL1 cells did not inhibit tumor growth in adoptive recipients (see Fig 5 legend for statistical analysis).
These results indicate that DLCs do not replicate initially as rapidly as wild-type BCL1 cells but that their replicative capacity is eventually fully restored in an adoptive recipient.
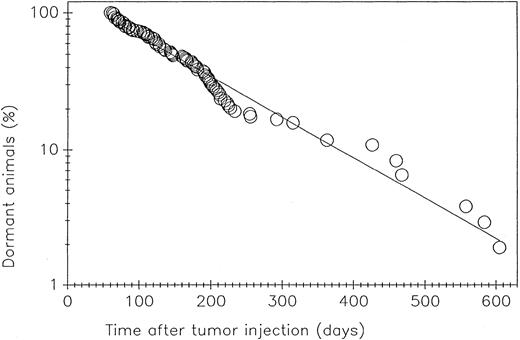
Kinetics of loss of dormancy.We have observed the natural history of dormant BCL1 tumor in 114 mice observed for 610 days after challenge with the BCL1 tumor cells. As shown in Fig 6, there was a steady rate of loss of dormancy over this period of time, suggesting a stochastic process. One likely interpretation is that genetic alterations had taken place in the replicating tumor cells.
The loss of dormancy with time after BCL1 challenge. One hundred fourteen dormant mice were examined weekly by palpation for splenic enlargement.11 The straight line was generated by computer analysis of the data; the regression coefficient is .985.
The loss of dormancy with time after BCL1 challenge. One hundred fourteen dormant mice were examined weekly by palpation for splenic enlargement.11 The straight line was generated by computer analysis of the data; the regression coefficient is .985.
Expression of mId and mIgM on escapee tumor cells.It might be predicted from studies of others13,14 26-30 that a major mechanism leading to escape of BCL1 cells from dormancy after anti-Id treatment is a loss in the expression of the mId because of the high mutation rate of genes encoding the hypervariable regions of the light and heavy chain. To investigate this, we phenotyped the splenocytes from dormant mice in which tumor had regrown. In such mice, progressive splenomegaly due to regrowth of the BCL1 tumor eventually results in the absorption of all the anti-Id from the sera of these mice. Thus, in the regrowing tumor cells, the Id epitopes are not masked by anti-Id antibodies and escapees can therefore be phenotyped for the expression of Id. An analysis of spleens from 46 animals showed the usual expression of Id and λ in 40 mice; there were 6 animals in which spleen cells were IgM−. Splenocytes from these mice were transferred to nonimmune recipients and in 2 of the 6 transfers, Id+ tumor cells grew in the secondary recipient spleens. This suggests that, in these 2 donor mice, anti-Id was either coating the Id+ tumor cells or had downregulated mIgM. Thus, only 4 of 46 mice had lost their Id due to lack of expression of mIgM.
Growth of Id+ escapee tumor cells adoptively transferred Into Id-immune mice.To confirm that Id+ escapees were variants that were resistant to the induction of dormancy by the presence of anti-Id antibodies in secondary recipients, such escapees were injected into Id-immune mice and the mice were followed for tumor growth. Table 1 summarizes the results of these experiments. Of 8 escapees from Id-immune BALB/c mice, 5 showed a significant loss of susceptibility to the induction of dormancy in Id-immune recipients. Dormancy has also been induced in SCID mice passively immunized with rabbit anti-μ and challenged with BCL1 cells.10 Two escapees from such passively immunized SCID mice (nos. 9 and 10) were not susceptible to the induction of dormancy when cells were injected into either Id-immune BALB/c mice or SCID mice passively immunized with rabbit anti-μ.
As shown in Table 1, escapees no. 4, 5, and 7 were still susceptible to the induction of dormancy when cells were reinjected into Id-immune mice, suggesting that changes in the host and not in the tumor cells were responsible for escape.
Anti-Id titers in mice with regrowing tumors.It was possible that anti-Id levels and/or cellular immunity were low in escapees no. 4, 5, and 7 and that a poor or waning immune response was responsible for the failure of the 3 mice to maintain the BCL1 cells in a dormant state. Titers of anti-Id could not be determined after growth of the DLCs, because the enlarged tumor cell population adsorbed antibody from the circulation. Therefore, to explore this question, we examined the anti-Id titers in Id-immune mice before challenge with BCL1 cells to determine whether there was a relationship between the levels of IgG anti-Id in response to the immunization regimen and the capacity of the immune host to induce dormancy in the BCL1 population.
As shown in Table 2, the presence of low serum anti-Id titers before BCL1 challenge increased the probability that the tumor would grow rapidly in such mice and that dormancy would not be induced. A higher anti-Id titer correlated with an increase in the proportion of mice that became dormant. The differences between the nondormant and either of the dormant groups is highly significant. In dormant mice observed for the time of onset of splenomegaly (line 3; the other dormant groups were killed for other experimental studies), the duration averaged 132 days, compared with 36 days in the nondormant mice. Thus, the level of anti-Id and, possibly, the level of cellular immunity (which was not assessed) could play a role in the induction and maintenance of dormancy.
Analysis of BCL1 escapees.Of the 10 BCL1 escapees described in Table 1, 7 were verified as variants, ie, they would grow when adoptively transferred to Id-immune mice. These included no. 1, 2, 3, 6, 8, 9, and 10. In contrast, escapees no. 4, 5, and 7 were not variants and failed to grow when adoptively transferred into Id-immune mice. All 7 variants examined were Id+, as determined by positive staining with the RAId, and yet were growing in the presence of anti-Id, suggesting that there was a defect in the ability of anti-Id to signal growth arrest. Because these variants were Id+, we hypothesized that the BCL1 cells had become altered in such a way that anti-Id could no longer signal growth arrest. To explore this possibility further, we used Western blots to identify several molecules involved in anti-Ig signaling pathways, ie, Lyn, Syk, and HS1.
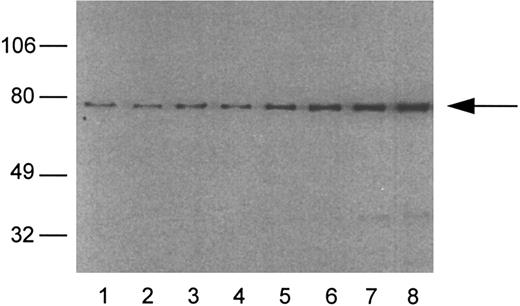
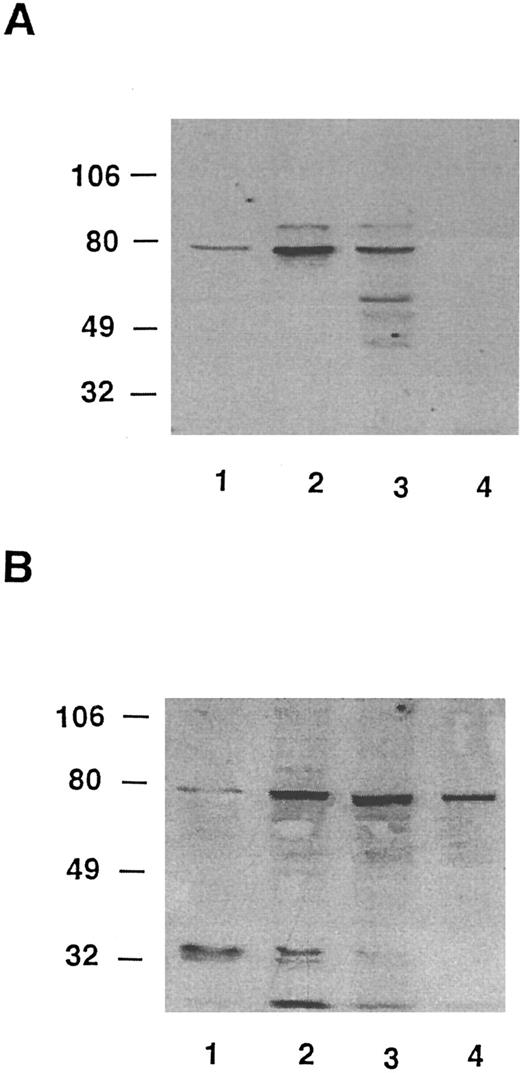
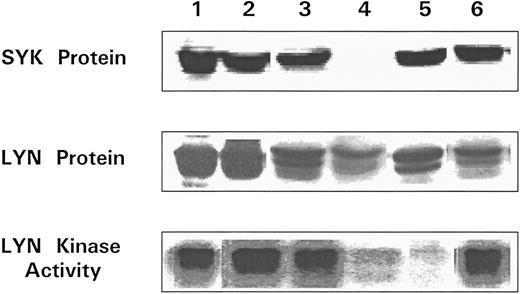
Lyn has been implicated in anti-Ig–mediated cell cycle arrest (CCA) of BCL1 cells24 and Syk is involved in the signaling of normal B cells.31 HS1 is a substrate for Lyn21,32 and it has been reported that HS1 is lacking in two variants of a murine B-lymphoma cell line that could not be negatively signaled by anti-μ in vitro.19 IgM-mediated apoptosis was also restored in one of the variants of the murine HS1-deficient B-cell lines by a retroviral expression vector for human HS1.19 To determine whether variants had normal levels of HS1, we used a Western blotting assay that could detect HS1 in BCL1 cells but little in normal spleen cells (Fig 7). BCL1 cells were mixed with normal splenocytes, and the lysates were immunoblotted with RbAHS1.19,20 As shown in Fig 7, the assay could detect HS1 in a mixture of 5% BCL1 cells and 95% normal splenocytes. Using this sensitive assay, lysates from 5 escapees (30% to 80% Id+ cells) and wild-type BCL1 cells (40% to 70% Id+ cells) were immunoblotted with either the RbAHS1 or MAHS1.19-21 Although all lysates were positive in the RbαHS1 blot, one escapee (no. 6) did not blot positively with MAHS1 (Fig 8B). These results suggest that there is a genetic variation in HS1 that affected the epitope detected by the MoAb but not the rabbit antibody. The putative HS1 variant (no. 6) also had no Syk protein detectable by the rabbit antibody (Fig 9).
An analysis of HS1 protein in mixtures of BCL1 cells and normal spleen cells. The percentage of BCL1 cells mixed with spleen cells: lane 1, 0%; lane 2, 2%; lane 3, 5%; lane 4, 10%; lane 5, 20%; lane 6, 30%; lane 7, 40%; and lane 8, 50%. The arrow indicates the position of HS1 at 75 kD. The integrated optical densities of the HS1 band are as follows: lane 1, 5.6; lane 2, 5.9; lane 3, 10.8; lane 4, 10.6; lane 5, 18.9; lane 6, 26.2; lane 7, 37.1; and lane 8, 46.2.
An analysis of HS1 protein in mixtures of BCL1 cells and normal spleen cells. The percentage of BCL1 cells mixed with spleen cells: lane 1, 0%; lane 2, 2%; lane 3, 5%; lane 4, 10%; lane 5, 20%; lane 6, 30%; lane 7, 40%; and lane 8, 50%. The arrow indicates the position of HS1 at 75 kD. The integrated optical densities of the HS1 band are as follows: lane 1, 5.6; lane 2, 5.9; lane 3, 10.8; lane 4, 10.6; lane 5, 18.9; lane 6, 26.2; lane 7, 37.1; and lane 8, 46.2.
Western blot analysis of HS1 in escapees. (A) MoAb AHS1. (B) RbAHS1. Lanes were loaded with equal amounts of cell lysates prepared from the same number of cells. Lane 1, 10% BCL1 cells plus 90% normal splenocytes. Lane 2, wild-type-BCL1 tumor. Lane 3, escapee no. 3. Lane 4, escapee no. 6.
Western blot analysis of HS1 in escapees. (A) MoAb AHS1. (B) RbAHS1. Lanes were loaded with equal amounts of cell lysates prepared from the same number of cells. Lane 1, 10% BCL1 cells plus 90% normal splenocytes. Lane 2, wild-type-BCL1 tumor. Lane 3, escapee no. 3. Lane 4, escapee no. 6.
Analysis of Lyn and Syk proteins and Lyn kinase in escapees. All lysates were prepared from splenocytes recovered from BCL1 tumor bearing nonimmune mice and BCL1 escapees. The presence of Syk (top) and Lyn (center) were determined by immunoblotting. Lyn tyrosine kinase was immunoprecipitated from separate samples and immune complex kinase assay performed (see the Materials and Methods) (bottom). Lane 1, BCL1 ; lane 2, escapee no. 1; lane 3, escapee no. 3; lane 4, escapee no. 6; lane 5, escapee no. 9; and lane 6, escapee no. 10.
Analysis of Lyn and Syk proteins and Lyn kinase in escapees. All lysates were prepared from splenocytes recovered from BCL1 tumor bearing nonimmune mice and BCL1 escapees. The presence of Syk (top) and Lyn (center) were determined by immunoblotting. Lyn tyrosine kinase was immunoprecipitated from separate samples and immune complex kinase assay performed (see the Materials and Methods) (bottom). Lane 1, BCL1 ; lane 2, escapee no. 1; lane 3, escapee no. 3; lane 4, escapee no. 6; lane 5, escapee no. 9; and lane 6, escapee no. 10.
The data in Fig 9 also show that all 5 of the variants tested (no. 1, 3, 6, 9, and 10 in Table 3) had readily detectable Lyn protein, but no. 6 and 9 had markedly reduced Lyn kinase activity as measured by autophosphorylation. Thus, as summarized in Table 3, at least 2 of 5 Id+ escapees examined had detectable alterations in one or more of the three proteins involved in signaling pathways that we studied. The other 3 escapees that were genetic variants could have alterations in other signaling proteins or in Syk kinase activity.
DISCUSSION
In the present study, an immunologic murine model of dormancy was used. Mice were actively or passively immunized so that there were circulating levels of anti-Id or anti-μ antibody. Id+ IgM+, BCL1 cells were then injected into the immunized mice and dormancy was induced in 70% of the recipients. As documented previously,12 dormancy can last for up to 2 years. The major findings to emerge from this study are as follows. (1) The population of DLCs in the spleen was stable for the 210 days of observation. (2) The DLCs were physiologically different from the wild-type DLCs because they grew more slowly for a period of time when transferred to naive recipients. (3) Eventually the DLCs regained most, if not all, of their malignant potential. (4) The kinetics of loss of dormancy occurred at a steady rate during the 2 years of observation. (5) Mice with high levels of serum anti-Id before BCL1 challenge were more likely to become dormant than those with lower levels of anti-Id antibody. (6) About 90% of the escapees were IgM+ and Id+; 10% were Id−IgM−. Thus, the majority of escapees were Id+ variants with decreased or no susceptibility to anti-Id–mediated induction of dormancy. (7) One of the five Id+ variants tested lacked an HS1 epitope, had no detectable Syk protein, and had markedly reduced Lyn kinase activity. A second Id+ variant had normal levels of HS1, Syk, and Lyn proteins but had markedly reduced Lyn kinase activity.
There is increasing evidence that tumor cells leave the primary site early in the course of the disease and that their presence is not incompatible with long-term survival or cure. However, in the follicular form of non-Hodgkin's lymphoma (NHL), conventional treatment can induce long-term remissions, but DLCs are invariably present and virtually all patients eventually die of the disease.26,33,34 The use of the polymerase chain reaction for lymphomagenic translocations of the VH of the tumor Ig indicate that patients with NHL and several other hematopoietic malignancies have detectable tumor during long-term remission or cure.35-41 For example, in B-cell acute lymphocytic leukemia in childhood, 16 of 17 patients had residual tumor after combination chemotherapy and many carried tumor 6 months to 1 year later and about half of these patients could be cured without further therapy.42 Similar observations have been made in T-cell acute leukemia in children43 and in chronic myelogenous leukemia.44 Even more striking examples of dormancy can be observed in melanoma and breast cancer, where there appears to be a steady rate of recurrence 10 to 20 years after the removal of a primary breast carcinoma and the recurrent tumor frequently grows at a rapid rate.2,3 45 Despite the clinical importance of tumor dormancy, little is known about the changes that take place in the host and/or the tumor cells that allow regrowth (escape) from the dormant state.
A provocative finding to emerge from studies of our model is that, although only a portion of the DLCs are cell cycle arrested,11,12 the population of DLCs is relatively stable for the 7 months of observation. One might have predicted that it would be unlikely that the rate of cell death induced primarily via antibody-mediated apoptosis10 and the rate of replication (a log2 function) would be similar, ie, tumor cells might disappear completely in some animals and grow very rapidly in others. However, there appears to be a balance between the rate of replication and cell death in many of the mice studied. The mechanisms underlying a possible coupling of the antitumor activity of the host with the ability of a subset of tumor cells to replicate are not known. Presumably, there are strong homeostatic mechanisms that keep the proportion of B and T cells commensurate with the host needs, etc.46-48 Perhaps these homeostatic mechanisms together with the immune response are responsible for the stability of the DLC population for many months and/or the CCA and apoptotic pathways are interrelated in some manner. However, regardless of the mechanisms involved, these studies indicate that a tumor cell population was dormant and that neither absence of tumor cells nor very slow growth was responsible for the clinically dormant state. Thus, our operational definition of dormancy is now superseded by formal proof of dormancy.
The present studies indicate that the DLCs eventually express their malignant potential. Thus, there were no significant differences between graded numbers of wild-type and DLCs in transferring progressive tumor growth to nonimmune syngeneic recipients. As few as three DLCs transferred progressive tumor growth to a syngeneic recipient. This result distinguishes the antitumor effect of anti-Id from gene therapy. Anti-Id suppresses the malignant phenotype by signal transduction mechanisms that override the genetic lesions that lead to neoplasia. When the anti-Id is no longer present, the cells can eventually express their malignant phenotype. However, it is provocative that the time at which splenomegaly occurred in these adoptive transfer experiments with DLCs was delayed compared with wild-type BCL1 cells. Thus, at all doses, the DLCs grew more slowly in the syngeneic naive mice than a similar number of wild-type BCL1 cells. This was not due to transfer of Id-immune T or B cells because the addition of such cells to wild-type BCL1 cells had no inhibitory effect. Our tentative conclusion is that there is a transient change in the physiology of these cells induced by their constant interaction with antibody (and Id-specific T cells). The mechanisms underlying this delay in growth are not known. Two possibilities include DNA damage that must be repaired and/or lack of growth factor receptors that must be upregulated.
The steady rate of loss of dormancy over a period of almost 2 years suggests a stochastic process, perhaps involving mutation of a protein in a signaling pathway. We have shown that, in the majority of escapees, alterations in the tumor cells rather than the host were responsible for escape from dormancy. However, we have also shown that changes in the host can probably lead to escape. Thus, we provide evidence to suggest that, in a small percentage of the mice with low anti-Id titers, the tumor regrows. For example, in mice that develop dormancy, the average serum anti-Id concentration before the injection of BCL1 cells was 358 μg/mL, whereas nondormant mice had an average concentration of 70 μg/mL. In addition, the duration of dormancy was higher in mice with higher anti-Id titers. This could be explained by the need of mIg hypercross linking for maximum negative signaling.49 The more effective the antibody level is in reducing the total number of cycling tumor cells via apoptosis and/or cell cycle arrest, the less likely the probability for mutations.
Ten percent of the escapees and their progeny were mIg− after transfer to nonimmune mice, suggesting that a mutation had occurred either in the genes encoding the heavy or light chain or one of the proteins that allow surface expression (eg, BIP50). At first glance, these results appear to be different from those reported by Meeker et al26 in which monoclonal anti-Id was used to induce regression of NHL in humans. In their studies, Id− variants regrew in many treated patients presumably because of the high rate of mutation in the genes encoding the Ig hypervariable regions, as mentioned above. Our results indicate that, in the presence of polyclonal anti-Id, Id−, IgM+ variants did not appear, presumably because it is improbable that several (or more) idiotopes would be altered by mutation in the same cell. An additional important mechanism to explain our data versus those of Meeker et al26 is our recent finding that monoclonal anti-IgM antibodies are frequently ineffective at inducing apoptosis and CCA in a human lymphoma (Burkitt's) cell line.49 However, when three anti-μ MoAbs, recognizing three different epitopes, were used simultaneously in vitro, they were effective at inducing both.49 Hence, for clinical use, it may be important to use a mixture of monoclonal anti-Id antibodies. Indeed, Levy et al27 (personal communication, 1996) have used such a mixture of MoAbs in the treatment of NHL.
In the 90% of escapees that were BCL1 -Id+, other components of the signaling cascade may have undergone genetic variation. Thus, in the limited survey performed here, alterations in Syk, Lyn, and HS1 are suggested by either the loss of an epitope recognized by an MoAb or loss of functional kinase activity. In this regard, prior studies of others have underscored the importance of Lyn, Syk, HS1, and other members of the signaling cascade in conveying signals from mIgM to the nucleus.21,32,51-64 Our own studies using antisense oligonucleotides have implicated Lyn in IgM-mediated CCA.24 Analysis of the DT-40 chicken B-cell Syk− cell line provides strong evidence that Syk is essential for IgM-mediated apoptosis.65 Although Lyn, Syk, and HS1 are candidates for mutations, there are many other molecules in the signaling cascade that could also be responsible for loss of susceptibility to the induction of anti-Id–mediated dormancy, eg, PIP2, PLCγ, MAP kinase, BLK, etc.66-75 The molecular basis for these changes is currently under study in cloned cell lines established from these escapees.
With regard to cell signaling, studies by Brown et al34 and Vuist et al76 of NHL patients are particularly pertinent because they have observed a correlation between the capacity of anti-Id to induce phosphorylation in vitro in neoplastic B cells obtained by biopsy and clinical remission in patients.76 This suggests that the signaling capacity of antibody may be essential for effective antitumor activity. This clinical observation is in accord with our conclusions from in vivo and in vitro studies using the BCL1 model in which the critical role of signaling in the induction of dormancy has been proven. Another pertinent observation by this group is that vaccination of NHL patients with the patient's tumor Ig coupled to KLH given in threonyl muramyl dipeptide adjuvant77 78 stimulated an Id-specific humoral or cellular immune response in about half the patients and the majority of such patients do not relapse for periods of up to several years. From the group that did not develop an immune response, the majority have relapsed.
In the experiments described here, the role of Id-specific cellular immunity was not investigated; our prior studies indicate that the major effector pathway in induction of dormancy is mediated by signaling antibody. However, this does not exclude a role for Id-specific T-cell immunity or nonspecific effector functions of the antibodies. With regard to cellular immunity, prior studies have indicated that allogeneic bone marrow transplantation can induce tumor dormancy79-81 and that partial CCA is induced.11,79 Moreover, T cells from Id-immune mice can significantly enhance the induction and maintenance of dormancy induced by passively transferred anti-Id antibody in SCID mice.10 Additionally, Demanet et al82 have shown that both CD4+ and CD8+ cells are required in the successful treatment of BCL1 in vivo with an anti-BCL1Id/anti-CD3 bispecific antibody. Morecki et al83 have presented data consistent with a contribution of cell-mediated immunity in protecting mice immunized with irradiated BCL1 tumor from a subsequent tumor challenge. Hsu et al84 have shown that 3 of 4 patients vaccinated with antigen-pulsed dendritic cells develop cellular responses and undergo tumor regressions. Therefore, it is possible that, when cellular immunity is studied in escapees, a poor Id-specific cellular immune response could also contribute to escape. With regard to effector functions of antibody, eg, ADCC, there is formidable evidence that these functions can contribute in a major way to antibody-induced tumor immunity in general.85 Hence, a dynamic equilibrium may exist between the tumor cells and the several mechanisms by which the immune response inhibits tumor growth and maintains dormancy.
ACKNOWLEDGMENT
We thank Y. Chinn for expert technical assistance and C. Patterson and S. Chadwick for expert secretarial assistance. We also thank J. Bolen for the GST-lyn fusion construct. We are particularly indebted to Dr D. McIntire for his help in statistical analysis.
Supported by National Institutes of Health Grants No. CA-28149 and CA-58321, a grant from The Meadows Foundation, and American Cancer Society Grant No. IM-767.
Address reprint requests to Dr Ellen S. Vitetta, Cancer Immunobiology Center and Department of Microbiology, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas, TX 75235.


![Fig. 3. Progressive tumor growth in nonimmune versus Id-immune mice challenged with BCL1 tumor cells. (A) The spleens from naive (○) and Id-immune (•) BALB/c mice injected with 106 BCL1 cells were palpated as described11 on the days indicated. Naive mice have an average spleen size of ∼0.5. Immunization with BCL1 Id results in a spleen size of 1 to 1.5 before BCL1 tumor injection. (B) BCL1 cells (1 × 106) were injected into naive BALB/c mice (○) or into Id-immune BALB/c mice (•). At the times indicated, mice were killed and the number of BCL1 tumor cells was determined by staining with RtABCL1Id for naive animals or RtAMλ for Id-immune animals (endogenous mouse anti-BCL1 Id [MA BCL1 Id] blocks staining with RtABCL1-Id but not the RtAMλ Ab) and analyzed by FACS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4425/3/m_bl_0037f3.jpeg?Expires=1763596949&Signature=MWtSJ6aXZmZXkk-v0MV6JISJM-350fKcZOuqyUyMOJ7lTuJ62dVDfJmOViyAix09YWSbKnFbpZGBZX~VV0OYNe0lpdvJQ1uhNApmkhdX6Q7oi6w4GUn1WRv2avAysjDvJSFpth2y8LpKdoFcDzs7b6qfT2pGpPh1~TpdvXvX0yIdSvHSdI2Kk9T-qgY9ay-MA9bCCNgmo3jto3KzgkLuE8haBbcO2x63C3l7DkbW~h~YgR8lVKGlrbexGO6GF4TognWNvbpzBQ4QVKaJGUm9m2~9lF677OetpkSaKCeFZrH7ipNJT5MtnLOLtFCuG8Rp1oIb~Ns~ul85Jq5Iu04yyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)