Abstract
Clinical modalities based on inhibition of gelation of HbS are hindered by the lack of quantitative information on the extent of participation of different amino acid residues in the aggregation process. One such site is Asp-85(α), which is involved in a parallel interdouble strand ionic interaction with Lys-144(β) according to the crystal structure of HbS, but electron microscopy does not specifically show Asp-85(α) as a contact site for fiber formation. Using a yeast recombinant system, we have substituted this site by Lys to abolish ion pairing and to make a quantitative determination of its participation in aggregation. The purified double mutant was shown to have the expected pI, the calculated molecular weight, correct amino acid composition, and peptide map. The recombinant double mutant has an oxygen affinity of 10 mm Hg, which is identical to that for HbA and HbS under the same conditions; it also has high cooperativity with an average n value of 2.7. The change in P50 in response to chloride ions was about 25% less than that for HbA or HbS and is ascribed to the introduction of a new positive charge near one of the major oxygen-linked chloride binding sites of hemoglobin. The gelation concentration of the double mutant was measured by a new procedure (Bookchin et al, 1994); the maximal amount of soluble hemoglobin (Csat ) in the presence of dextran indicated a decreased tendency for gelation with a Csat of 53 mg/mL compared with 34 mg/mL for HbS. This inhibitory effect is smaller than that of the E6V(β)/L88A(β) (Csat , 67 mg/mL) and the E6V(β)/K95I(β) (Csat , 90 mg/mL) recombinant hemoglobins. Thus, we would classify Asp-85(α) as a moderate contributor to the strength of the HbS aggregate. This wide range of gelation values demonstrates that some sites are more important than others in promoting HbS aggregation.
THE PRIMARY CAUSE for sickle cell anemia has been known since 1956 when Ingram1 showed that the difference in electrophoretic behavior between HbS and HbA described by Pauling et al2 was due to the replacement of a single amino acid, ie, Glu → Val at position 6 of the β-chain E6V(β). This point mutation leads to the initial strong hydrophobic interaction between the adjacent Hb tetramers as described in detail by Bunn and Forget.3 The subsequent formation of long Hb fibers involves many sites of contact as deduced initially from the effects of natural Hb mutants with substitutions at other sites on the polymerization of HbS4 and subsequently from the x-ray structure5,6 and also by electron microscopy,7,8 although differences in interpretation of these models persist. Because this structural information is available, it is now feasible to study the overall strength of various sites in sickle hemoglobin aggregates.9,10 An alternate approach, ie, chemical modification has provided information about the extent of participation of other amino acid residues in the aggregation process.11-15 However, with this approach there are many sites that are not accessible to a given reagent. More recently, the use of recombinant Hb16,17 has provided the advantage that any amino acid substitution can be made at sites not represented by the natural mutants or that cannot be modified by chemical procedures. Indeed, results obtained with both natural and recombinant Hbs might resolve differences between models of the HbS polymer.7 8
Most of the earlier work with the recombinant Hb system focused on the sites at or near the hydrophobic pocket of the beta chain, which is the acceptor for the mutated Val residue.18-20 Recently, we showed that a residue located at the exterior of the Hb tetramer, (Lys-95[β]),21 reduces the gelation nearly twice as much as a residue in the hydrophobic pocket, (Leu-88[β]).20 This finding was in agreement with the electron microscopy studies,8 but not with the crystal structure of HbS,6 which shows no contacts between Lys-95(β) and the amino acid side chains of adjacent tetramers. Because the Lys-95(β) site is on the exterior of the Hb tetramer, it is a potentially accessible site for compounds to be targeted for inhibiting gelation, whereas the residues at the hydrophobic pocket are buried within the Hb tetramer and hence not likely candidates.
The studies presented in this report represent a continuation of a systematic effort that we initiated recently employing a yeast expression system for producing recombinant HbS double mutants.20,21 These recombinant hemoglobins, which have been characterized by a number of biochemical criteria, are being used to obtain quantitative information on the participation of particular amino acid side chains in the gelation process to identify potential target sites for therapeutic interventions. In this report, we describe an HbS double mutant where the second mutation is on the alpha chain, Asp-85(α). Although several α- and β-chain sites have been reported to participate in gelation through the study of natural mutants,22-24 no information exists on α-chain recombinant mutants. The Asp-85(α) site described in this study was chosen because the extent of its participation in gelation has not yet been determined. The electron microscopic studies8 indicate that Asp-85(α) is 5 to 8 A away from an adjacent HbS tetramer, suggesting that it might be a contact site of moderate strength in the aggregate. The crystal structure of deoxy HbS also implicates Asp-85(α) in interdouble strand contacts,5 6 as it has several interactions including an ion pairing with Lys-144(β). To quantitate its role in aggregation, we replaced Asp-85(α) with Lys by site-directed mutagenesis to completely abolish its ion interaction with Lys-144(β). However, in view of a possibly unfavorable interaction between Lys-85(α) and Lys-144(β), it was of critical importance to establish that the functional integrity of the double mutant was not compromised. For measuring the gelation concentrations, we have adapted a new and sensitive procedure to make quantitative comparisons between E6V(β)/D85K(α) and previously produced HbS double mutants and to evaluate its significance as a potential target site for the development of chemical inhibitors against HbS gelation.
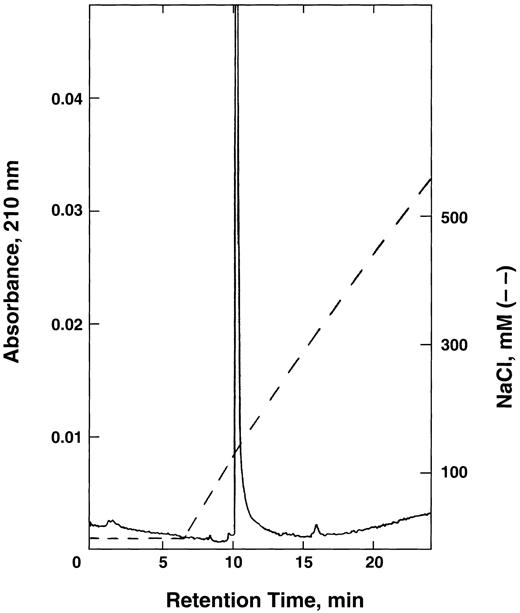
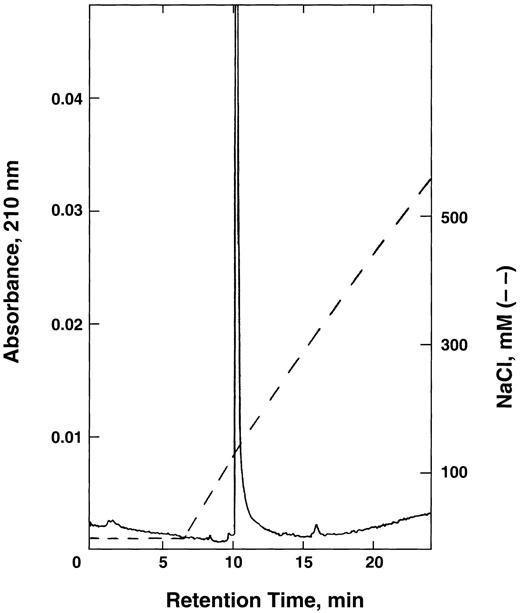
FPLC chromatography of E6V(β)/D85K(α) double mutant on Mono Q column. The hemoglobin obtained from the yeast extract was first purified on CM-52 cellulose as described in the text, applied to Mono Q column, and eluted using a linear NaCl gradient in 20 mmol/L Tris-acetate buffer, pH 8.0.
FPLC chromatography of E6V(β)/D85K(α) double mutant on Mono Q column. The hemoglobin obtained from the yeast extract was first purified on CM-52 cellulose as described in the text, applied to Mono Q column, and eluted using a linear NaCl gradient in 20 mmol/L Tris-acetate buffer, pH 8.0.
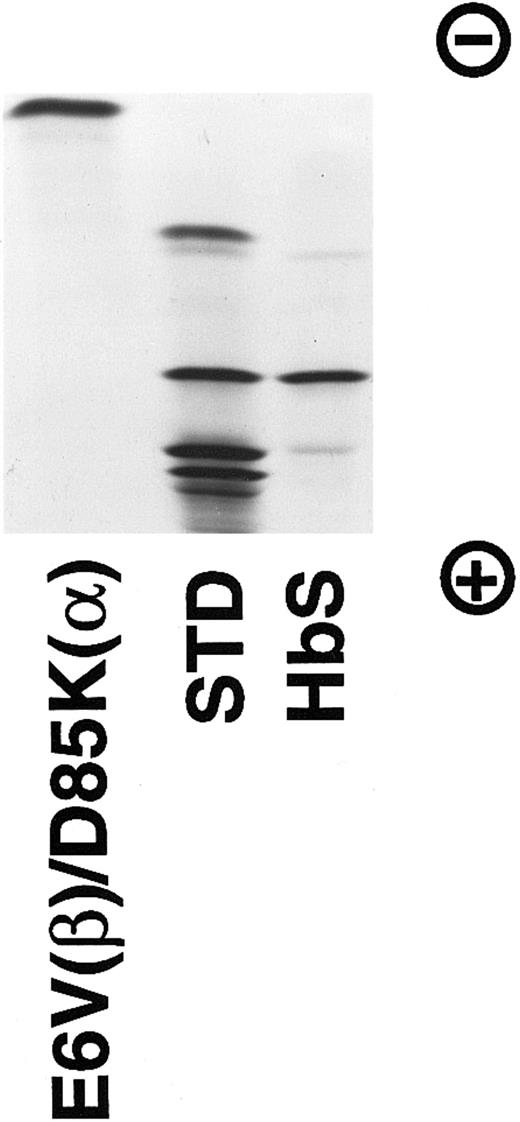
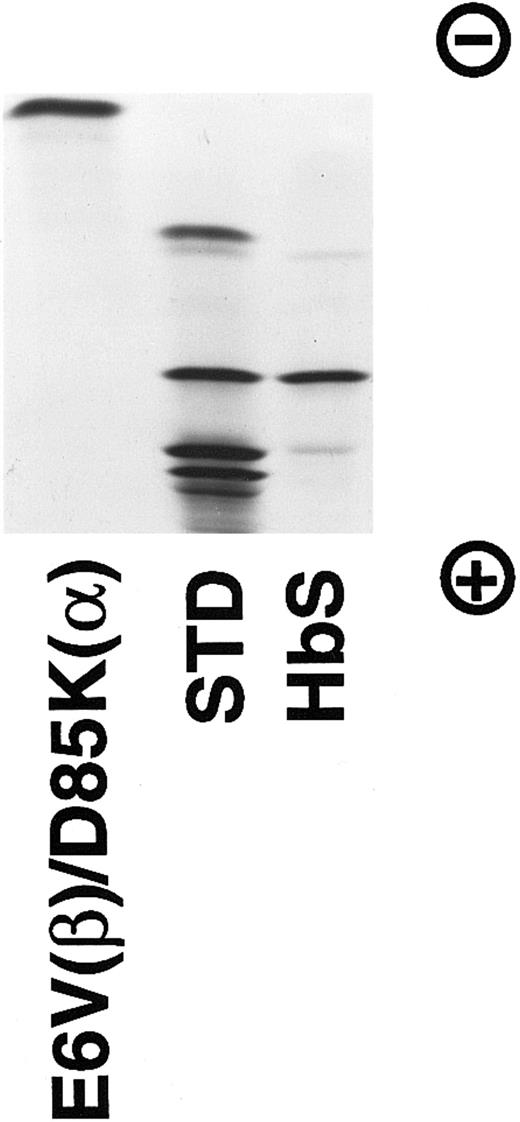
Isoelectric focusing of the purified E6V(β)/D85K(α) Hb. A gel from Isolab (pH 6-8) containing about 30 μg of protein was electrophoresed at 10 W for 45 minutes and stained by bromophenol blue. The standard contains (from top to bottom): HbC, HbS, HbF, and HbA.
Isoelectric focusing of the purified E6V(β)/D85K(α) Hb. A gel from Isolab (pH 6-8) containing about 30 μg of protein was electrophoresed at 10 W for 45 minutes and stained by bromophenol blue. The standard contains (from top to bottom): HbC, HbS, HbF, and HbA.
MATERIALS AND METHODS
Reagents and plasmids.The restriction endonucleases, T4 polynucleotide kinase, alkaline phosphatase, DNA ligase, and Gene 32 Protein, T4 were from Boehringer Mannheim (Germany). The DNA sequencing kit and the T7 DNA Polymerase (Sequenase Version 2.0) were obtained from US Biochemical Corp (Cleveland, OH). The 35S-labeled deoxyadenosine triphosphate (dATP) was from DuPont NEN (Boston, MA). The oligonucleotides were synthesized by Operon Technologies (Alameda, CA). CM-Cellulose 52 was from Whatman, MonoQ from Pharmacia (Stockholm, Sweden), and high-performance liquid chromatography (HPLC) columns (C-4 and 400VHP575) from Vydac (Southborough, MA). Tosylphenylalanine chloromethylketone (TPCK)-treated trypsin, dextran, diphosphoglycerate (DPG), and inositol hexaphosphate (IHP) were purchased from Sigma (St Louis, MO). pBluescript II SK(+) was from Stratagene (La Jolla, CA). The construction of pGS189 and pGS389 plasmids is described elsewhere.16 21 All other reagents were of analytical purity.
Site-directed mutagenesis.To prepare the E6V/D85K(α) mutant, we first inserted the α-globin-coding gene from pGS189 to pBluescript II SK(+) as a Sal I fragment. This fragment contains the full-length human α-globin cDNA under transcriptional control of a pGGAP promoter. The modified plasmid (pSK[+]α) was transformed in Escherichia coli BW313, and the uridine-containing single-stranded DNA was isolated from the supernatant of the bacterial culture after infecting the cells with M13KO7 helper phage. The oligonucleotide 5′-GTGCGCGTGCAGCTTGCTCAGGGCGGA-3′ was used to create the Asp-85 → Lys mutation by the method of Kunkel.25 The underlined bases are those used to create the desired mutation. The presence of the mutation was sought by screening with partial sequencing of the mutation site. The mutated α-globin region was subcloned into pGS189sickle, which contains the native α-globin and the 6(β)Glu → Val mutated β-globin cDNAs,17 by digestion with BssHII and BstEII enzymes to create incompatible cohesive termini and thus to increase the percentage of the insert in correct orientation. Finally, the α- and β-globin gene cassette was isolated as a Not I fragment after digesting the newly synthesized pGS189sickle-85(Lys) with Not I and Bgl I and inserted into pGS389 previously digested with Not I. The correct insertional direction was verified by restriction mapping and the entire α-globin gene was sequenced to establish that the Asp-85(α) → Lys was the only mutation.
Growth of yeast and purification of the mutant Hb.The yeast cells were transformed by the pGS389sickle-85(Lys) plasmid using a lithium acetate method.26 The transformants were selected and the copy number of the plasmid was increased by growing the yeast on a complete minimal medium first without uracil, then without uracil or leucine. To express the E6V(β)/D85K(α) mutant hemoglobin, the yeast was grown in yeast extract-peptone (YP) medium for 4 days with ethanol as the carbon source.16 The promoter controlling the transcription of the globin genes was induced by adding 3% galactose for 20 hours before harvesting of the yeast cells. The cells were disrupted in a Bead Beater homogenizer (Biospec Products, Bartlesville, OK) and the Hb double mutant was purified on a CM-Cellulose 52 column with a slight modification from Martin de Llano et al,17 as described below.
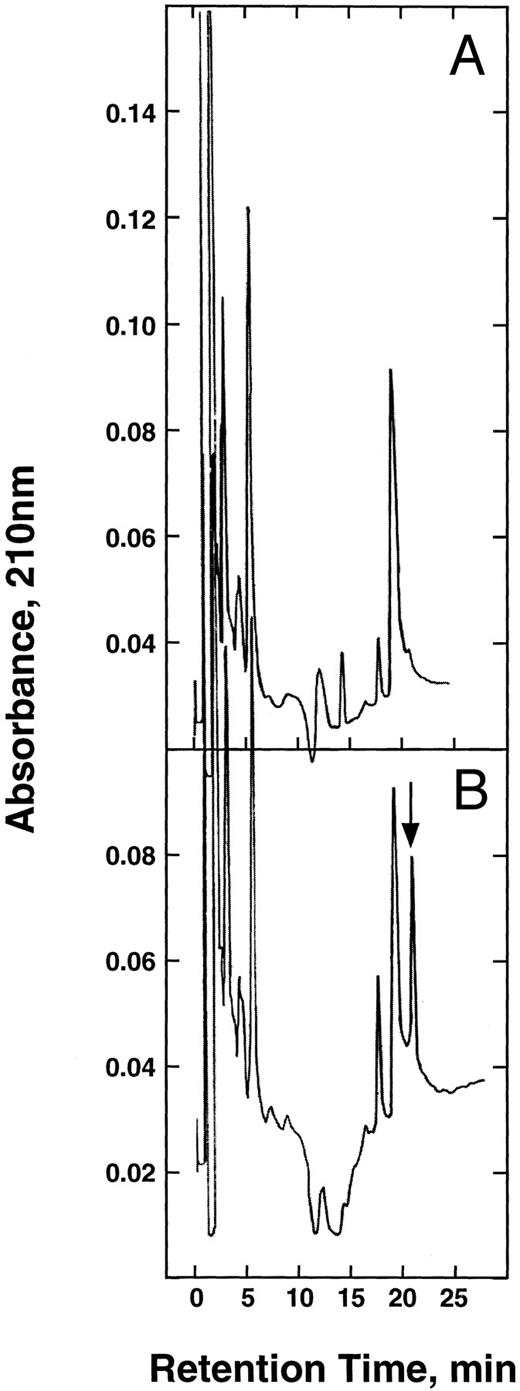
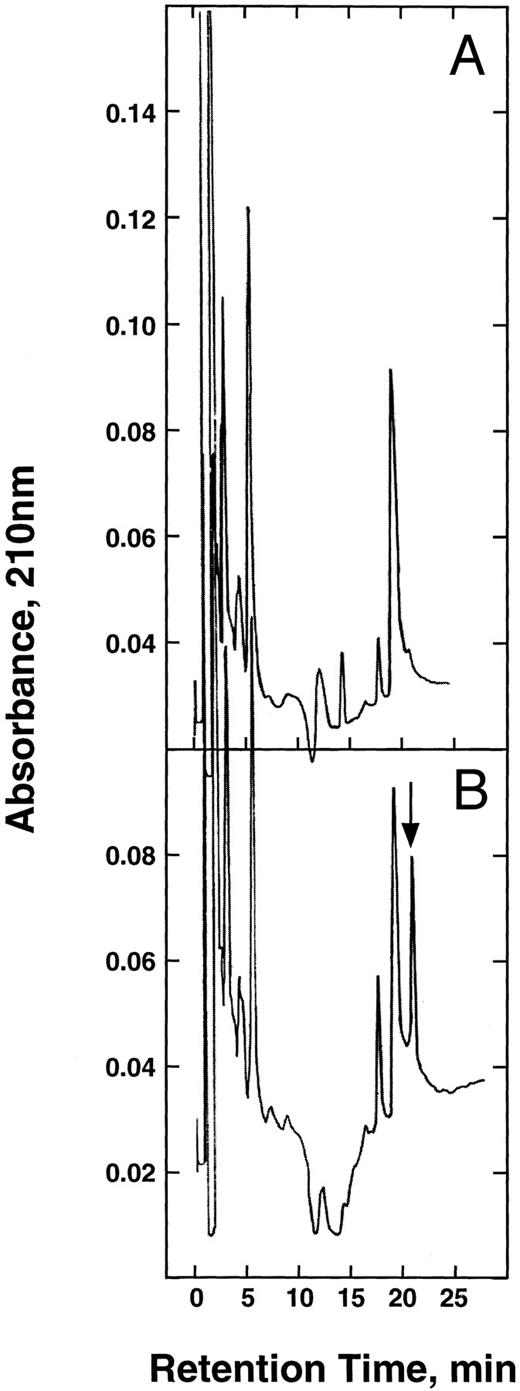
Tryptic peptide maps of the α-chains. The α-chains of HbA (A) and E6V(β)/D85K(α) (B) were isolated, carboxymethylated, and digested with trypsin as described in the text. The resulting peptides were separated on a Vydac 400VHP575 strong cation exchange column by NaCl gradient (0 to 100 mmol/L) in 20 mmol/L Na-acetate/10% acetonitrile buffer (pH 5.2). The arrow shows the mutant pentapeptide.
Tryptic peptide maps of the α-chains. The α-chains of HbA (A) and E6V(β)/D85K(α) (B) were isolated, carboxymethylated, and digested with trypsin as described in the text. The resulting peptides were separated on a Vydac 400VHP575 strong cation exchange column by NaCl gradient (0 to 100 mmol/L) in 20 mmol/L Na-acetate/10% acetonitrile buffer (pH 5.2). The arrow shows the mutant pentapeptide.
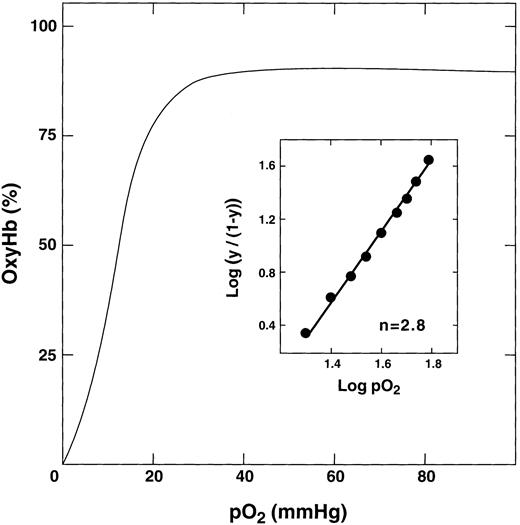
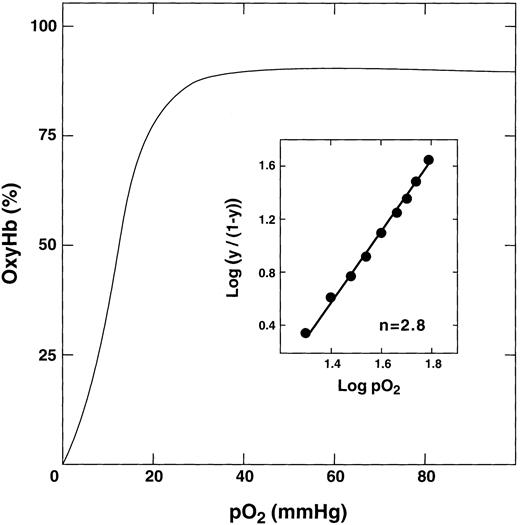
Oxygen binding curve of E6V(β)/D85K(α). The purified double mutant in 50 mmol/L bis-Tris acetate, pH 7.4, was concentrated to 0.5 mmol/L, converted to the oxy form, and the oxygen binding curve was measured at 37°C by a modified Hem O Scan instrument. The inset shows the calculation of the n value.
Oxygen binding curve of E6V(β)/D85K(α). The purified double mutant in 50 mmol/L bis-Tris acetate, pH 7.4, was concentrated to 0.5 mmol/L, converted to the oxy form, and the oxygen binding curve was measured at 37°C by a modified Hem O Scan instrument. The inset shows the calculation of the n value.
Analytical methods.Mass spectrometric analysis was done on a matrix-assisted laser desorption time-of-flight mass spectrometer constructed at Rockefeller University, New York, NY and described elsewhere.27,28 Fast Protein-Peptide-Polynucleotide Liquid Chromatography (FPLC) purification on Mono Q (Pharmacia, Uppsala, Sweden) was performed using 20 mmol/L Tris-acetate buffer, pH 8.0, and an NaCl gradient from 0 to 1 mol/L. Isoelectric focusing, amino acid analysis, and other procedures were performed as described earlier.20,21,29,30 To isolate the α- and β-globin chains, a Vydac C-4 column was equilibrated with 37.6% acetonitrile in 0.1% trifluoroacetic acid and the sample was eluted with a linear gradient of acetonitrile to 43.3%. The isolated α-globin chain was digested with trypsin,21 and the resulting peptides were separated on a Vydac 400VHP575 strong cation exchange column by a linear gradient of NaCl in 20 mmol/L sodium acetate +10% acetonitrile, pH 5.2.
Tetramer-dimer dissociation constant.This measurement was performed on the liganded recombinant Hb on Superose-12 using a Pharmacia FPLC system.31
Functional studies.The oxygen dissociation curves were determined at 37°C on a modified Hem O Scan instrument (Aminco, Silver Spring, MD) as described previously.21,29 Before the measurements, the Hb samples were dialyzed, converted to the oxy form,32 and concentrated using CentriPrep, Centricon and MicroCon ultrafiltration devices (Amicon; molecular weight cut-off of 10,000). The final protein concentrations were verified by amino acid analysis on a Beckman 6300 analyzer (Palo Alto, CA). When evaluating the effects of allosteric modulators, the samples were in 50 mmol/L bis-Tris buffer, pH 7.4.
For determining the Bohr effect, the Hb samples were first dialyzed against H2O, concentrated to a final concentration of 1 mmol/L and diluted with an equivalent amount of 100 mmol/L bis-Tris buffers of different pH values before the measurement of the oxygen dissociation curves. The final pH was verified by a microelectrode (Microelectrodes Inc, Bowdoinville, ME).
Determination of Csat . The method used for determining the gelation concentration (Csat ) of Hb is based on the decrease of the solubility of HbS in the presence of dextran.33 The E6V(β)/D85K(α) or HbS sample in the oxy form in 50 mmol/L potassium phosphate buffer, pH 7.5 was mixed with dextran (100 mg/mL final concentration). Mineral oil was then layered on top and fresh sodium dithionite solution (50 mmol/L final concentration) was added below the Hb-dextran mixture anaerobically using a gas-tight syringe. The reaction mixture was incubated at 37°C for 30 minutes, mixed thoroughly, and centrifuged in a microcentrifuge for 30 minutes. The clear supernatant was carefully separated from the aggregated Hb and its hemoglobin concentration measured by amino acid analysis on a Beckman 6300 amino acid analyzer. The difference between the Hb concentration of the supernatant (Csat ) and the initial Hb concentration is an indication of the extent of gelling.
Molecular modeling.The molecular modeling was done on a Silicon Graphics, Inc (Mountain View, CA) Power Indigo 2 computer using the molecular modeling program Insight II (Biosym/MSI). The coordinates of human sickle hemoglobin (1HBS) were obtained from the Brookhaven protein database.
RESULTS
Mutagenesis.Because no α-chain HbS mutants have been produced using the yeast expression system, it was necessary to create a plasmid containing the α-globin gene. This was accomplished by taking advantage of the unique Sal I site at the multiple cloning site of pBluescript II SK(+). A Sal I fragment of the pGS189 extending downstream from the end of the β-globin gene to the end of the α-globin gene was ligated with pBluescript II SK(+) previously digested with the same restriction enzyme. The single-stranded DNA rescued from this plasmid was used as a template in site-directed mutagenesis. Presumably the high G/C content of the oligonucleotide (see Materials and Methods), and its consequent strong tendency to form a hairpin loop (ΔG = −0.6 kcal mol−1) caused the mutation frequency to fall below the detection limit when using the standard Kunkel method.25 By decreasing the oligonucleotide:template ratio to 50:3 and performing the annealing reaction at a temperature change of 95°C to 20°C over a period of 15 hours in the presence of Gene 32 Protein, the open circular, incomplete circular, and the covalently closed circular double-stranded DNA forms were obtained by the extension reaction as shown by Yuckenberg et al34 (data not shown). The mutation frequency was increased to 25%.
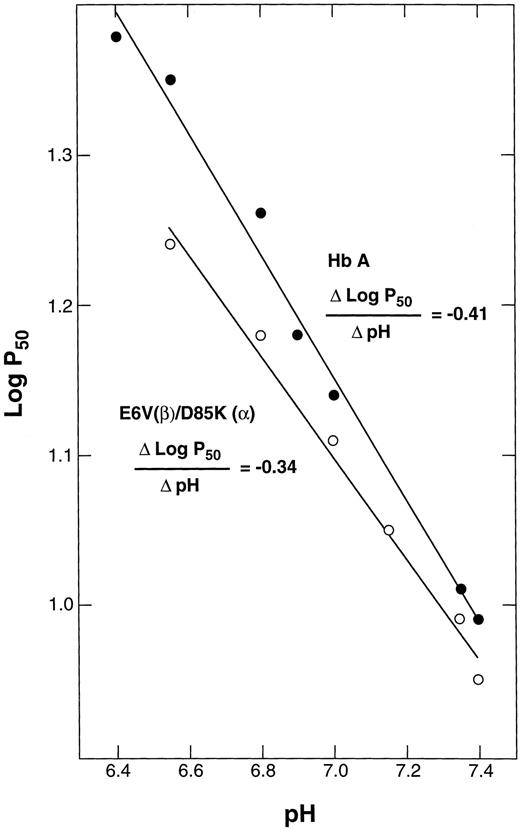
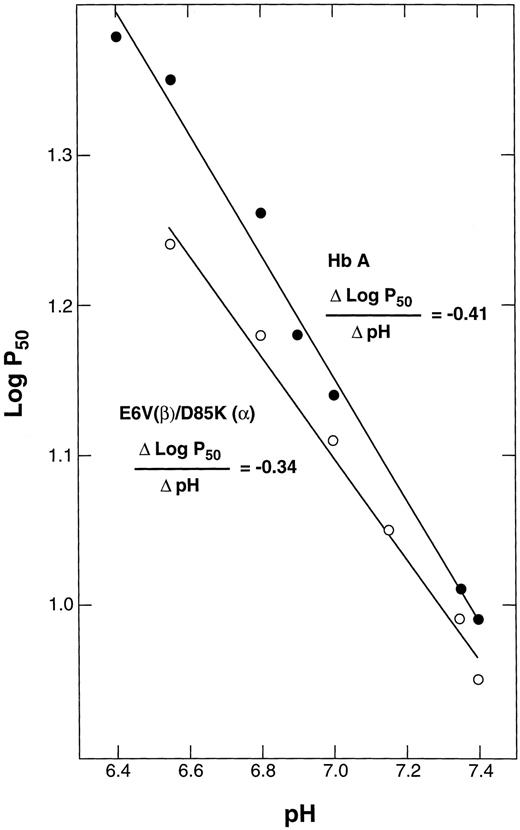
The alkaline Bohr effect of E6V(β)/D85K(α). The purified double mutant in oxy form was diluted with bis-Tris buffers of different pH values to a final concentration of 0.5 mmol/L Hb in 50 mmol/L bis-Tris, and the P50 values were determined as in Fig 4.
The alkaline Bohr effect of E6V(β)/D85K(α). The purified double mutant in oxy form was diluted with bis-Tris buffers of different pH values to a final concentration of 0.5 mmol/L Hb in 50 mmol/L bis-Tris, and the P50 values were determined as in Fig 4.
Purification.After subcloning the mutated DNA fragment into pGS389, which contains the human α- and β-globin cDNAs, the sickle hemoglobin double mutant, E6V(β)/D85K(α) was expressed in yeast as described in Materials and Methods. On a CM-Cellulose 52 column, it adhered more avidly than HbS, as expected for a mutant having an Asp to Lys surface mutation. For elution, a gradient of up to 25.5 mmol/L potassium phosphate instead of 15 mmol/L used for HbS17 was required.
The purified hemoglobin was rechromatographed on Mono Q column from which it eluted as a single peak (Fig 1) without any indication of multiple forms of recombinant hemoglobin reported earlier by others.35 36 Isoelectric focusing (Fig 2) of the purified E6V(β)/D85K(α) indicated an isoelectric point (pI)-value close to 8.0.
Gelation concentration (Csat ) of HbS and E6V(β)/D85K(α). Oxy hemoglobin samples in 50 mmol/L potassium phosphate, pH 7.5, were mixed anaerobically with dextran and sodium dithionite, incubated at 37°C, and centrifuged. The hemoglobin concentrations in the supernatant before (initial [Hb]) and after (equilibrium [Hb]) the incubation were determined by amino acid analysis. If the equilibrium [Hb] is lower than the initial [Hb], it represents the gelation concentration of Csat of the Hb.
Gelation concentration (Csat ) of HbS and E6V(β)/D85K(α). Oxy hemoglobin samples in 50 mmol/L potassium phosphate, pH 7.5, were mixed anaerobically with dextran and sodium dithionite, incubated at 37°C, and centrifuged. The hemoglobin concentrations in the supernatant before (initial [Hb]) and after (equilibrium [Hb]) the incubation were determined by amino acid analysis. If the equilibrium [Hb] is lower than the initial [Hb], it represents the gelation concentration of Csat of the Hb.
Mass spectrometry.Matrix-assisted laser desorption mass spectrometry was used to verify the molecular masses of the α- and β-globin chains of the purified Hb double mutant. The molecular mass (15,140.3) obtained for the α-chain by a time-of-flight method agrees well with the theoretical value of 15,139.5 mass units for the mutant α-chain. The difference of 13.9 mass units between the measured value and the calculated value of a wild-type α-chain of HbA (15,126.4 mass units) is close to the calculated difference (13.1 mass units) between the molecular masses of Asp and Lys residues. The molecular mass obtained for the β-chain (15,835.4 mass units) agreed with the calculated value for HbS (15,838.2 mass units) within the error of the measurement.
Peptide mapping.For this analysis, we took advantage of the creation of a new trypsin cleavage site at the 85-α position. Digestion of the isolated α-chain with trypsin produced the expected strongly basic pentapeptide (Leu-His-Ala-His-Lys). Because a reversed phase column generally used for peptide mapping was unable to separate this peptide from the others, we used a strong cation exchanger (Vydac 400VHP575) for this purpose. At pH 5, the pentapeptide has a net charge of +3 and separated as shown in Fig 3. In comparison, the chromatogram obtained using the α-chain of HbS lacked this peak. This pentapeptide was collected and it had the expected amino acid composition (Table 1).
Functional properties.The oxygen binding properties of E6V(β)/D85K(α) were determined under various conditions. In the absence of added chloride, the double mutant showed a typical sigmoidal oxygen equilibrium curve (Fig 4). The P50 value was 10, which is the same as that for HbA, HbS, and the K95I(β) recombinant Hb21 under the same conditions. The double mutant was cooperative with an average Hill coefficient of 2.7. In the presence of increasing amounts of chloride ions, its P50 value gradually increased to a maximum value of 17 mm/L Hg at a chloride concentration of 500 mmol/L (Table 2). Under similar conditions, we typically observe an increase in the P50 value of HbS, HbA, and other double mutants to a maximum value from 21 to 25 mm Hg.20,29,30 37 Thus, E6V(β)/D85K(α) shows a somewhat diminished response to chloride. A possible reason for this effect is discussed below.
The influence of two organic phosphate effectors, diphosphoglycerate (DPG) and inositol hexaphosphate (IHP), on the P50 value of the double mutant D95K was also tested (Table 2). Although the results in Table 2 show some variability with each Hb, with either allosteric effector the ratio of effector:Hb concentrations at the point of maximum effect was close to one for both the E6V(β)/D85K(α) mutant and HbS. The Hill coefficients of E6V(β)/D85K(α) in the presence of various concentrations of chloride or DPG varied between 2.3 and 2.8. Thus, the double mutant showed full cooperativity. Small conformational changes in these recombinant Hbs cannot be rigorously excluded. However, a careful circular dichroism study of another HbS double mutant20 did not show evidence for such an effect.
Tetramer-dimer dissociation constant.The tetramer-dimer dissociation constant for the liganded recombinant E6V(β)/D85K(α) was found to be 2.1 ± 0.2 μmol/L. This value is slightly higher than the 0.7 ± 0.2 μmol/L found for HbS, but because it is much less than the Hb concentration (500 μmol/L to -2 mmol/L) used for the functional studies such as the oxygen binding curve, the Bohr effect and the gelation studies, the double mutant was predominantly tetrameric during these measurements.
Bohr effect.When the pH was increased from 6.8 to 7.4, the P50 of the double mutant and of HbA decreased from 18.2 to 9.0. The slope of the line obtained by plotting the log P50 values against pH gives the alkaline Bohr coefficient (Fig 5). This value was calculated to be −0.34 (correlation coefficient, r = .994) for E6V(β)/D85K(α), which agrees reasonably well with the value of −0.41 (r = .995) obtained for HbA.
Gelation.The gelation concentration of E6V(β)/D85K(α) was measured in the presence of 100 mg/mL of dextran by the method of Bookchin et al33 as described in Materials and Methods. After incubation at 37°C and centrifugation, the maximum solubility (Csat ) of HbS or of the double mutant was obtained by measuring the Hb concentration in the supernatant. The Csat value of E6V(β)/D85K(α) at three different initial Hb concentrations of 56, 64, or 127 mg/mL was between 50 and 55 mg/mL (Fig 6). An initial Hb concentration of 31 mg/mL was below the Csat value and thus, no change in the Hb concentration after incubation and centrifugation was observed. The average Csat value of 53 mg/mL of E6V(β)/D85K(α) is clearly elevated compared with the value of 34 mg/mL of HbS, but much lower than the values for two other double mutants produced in our laboratory and whose gelation was determined by the same method, ie, E6V(β)/L88A(β) (67 mg/mL) and E6V(β)/K95I(β) (90 mg/mL).38
View along the central dyad axis of deoxy HbS. The distances between the two Lys-99 ε-NH2 groups, which is a major chloride-binding site in the center of the axis, and between the oxygen-linked chloride-binding residue (Val-1[α]) and the newly-created mutant residue (Lys-85[α]) are shown.
View along the central dyad axis of deoxy HbS. The distances between the two Lys-99 ε-NH2 groups, which is a major chloride-binding site in the center of the axis, and between the oxygen-linked chloride-binding residue (Val-1[α]) and the newly-created mutant residue (Lys-85[α]) are shown.
DISCUSSION
Recombinant hemoglobins have been produced using several different expression systems. In earlier studies, we showed that the recombinant sickle hemoglobin produced in the yeast expression system is indistinguishable by a number of chemical and biochemical assays from native HbS isolated from human red blood cells.29 The misfolding of hemoglobin reported for the E coli expression39 is not apparent for the hemoglobin expressed in yeast by any of the criteria that we have used for characterization. The production and characterization of an E6V(β)/D85K(α) hemoglobin double mutant is, to our knowledge, the first α-chain sickle Hb mutant produced using recombinant DNA technology. The mutant Hb was shown to have the predicted molecular mass, isoelectric point, and trypsin cleavage sites. Its oxygen affinity, response to DPG, Hill coefficient, and the alkaline Bohr effect were the same as the corresponding values for native HbA. Thus, it is identical to natural HbA and HbS in these properties. Possible minor local changes in the orientation of amino acids around the D85K(α) mutant site with respect to chloride binding are discussed below.
The replacement of Asp with Lys could have created strong unfavorable contacts between the α-85 site and some positively charged residues in its vicinity and thus have had a large effect on the intratetrameric contacts and on the functional integrity of the mutant hemoglobin. However, the dissociation constant (Kd) for the tetramer-dimer equilibrium of the E6V(β)/D85K(α) double mutant, measured by a chromatographic method developed in our laboratory,31 showed only a slightly increased Kd value (2.1 μmol/L) in comparison to HbS (0.7 μmol/L). For the functional properties of the double Hb mutant described in this study at a Hb concentration of 0.5 mmol/L to 2 mmol/L, the extent of dimerization of the double mutant is negligible.
The E6V(β)/D85K(α) double mutant had a slightly decreased response to chloride; in the presence of 0.5 mol/L NaCl, the double mutant had a P50 of 17 mm Hg in comparison to 21 mm Hg for HbS (ie, a 20% reduction in the oxygen-linked chloride effect). Earlier studies by us and others have shown that one of the oxygen-linked chloride-binding sites is located at the α-α interface in the central dyad axis of Hb and consists predominantly of amino acids Val-1(α) and Arg-141(α).40,41 As shown in Fig 7, the newly created D85K(α) mutation introduces the positive charge of Lys-85 10.8 Å apart from that of Val-1(α). In comparison, the distance between the two Lys-99 ε-NH2 groups in the center of the dyad axis, a major Cl− binding region,42-44 is 10.6 Å, small enough to be bridged by a Cl− ion with a Van der Waals radii of about 2.5 Å. Thus, the D85K(α) mutation could create a new Cl−-binding site that could compete with or diminish the oxygen-linked chloride effect at Val-1(α). Interestingly, Fronticelli et al45 have recently reported a construction of a human Hb mutant having a similar, but opposite, Cl− effect, where an A76K(β) mutation creates a new positively charged cleft between Lys-8(β) and Lys-76(β) and an increase in the effect of chloride ions on the oxygen affinity.
Three natural alpha-85 Asp mutants, Hb G-Norfolk (Asp → Asn),46 Hb Atago (Asp → Tyr),47 and Hb Inkster (Asp → Val)48 have been described. Each has an increased oxygen affinity, but no studies have been reported on their participation in gelation. Our results on the functional properties of the E6V(β)/D85K(α) mutant do not indicate an altered P50 and furthermore do not give any indication that the three-dimensional structure of the double mutant has been adversely affected by the substitution.
In this study, we have continued our efforts to understand not only the amino acid residues involved in the formation of HbS fibers, but also to measure the relative strength of these interactions in a quantitative manner. For this purpose, we have employed a new method33 for measuring the gelation concentrations of HbS variants, which takes advantage of the drastic diminishing effect of dextran on the solubility of HbS. Using this method, we showed that the gelation concentration of the E6V(β)/D85K(α) double mutant was elevated to 53 mg/mL as compared with 34 mg/m obtained for HbS. This decreased tendency for gelation is consistent with the x-ray studies,5 6 ie, in the HbS crystal the Asp-85(α) residue forms an ion pair with Lys-144(β) of the adjacent Hb tetramer, which is abolished in the D85K(α) mutant and the gelation is consequently inhibited.
Our conclusion that Asp-85(α) contributes moderately to the strength of the HbS aggregate is also consistent with, but does not distinguish between the electron microscopic models7,8 and furthermore establishes its quantitative participation in gelation. The model of Watowich et al8 suggests some degree of participation of Asp-85(α) in the gelation, as it is 5 to 8 Å apart from the adjacent tetramer. The model also shows that Lys-95(β) is involved in an intermolecular contact of less than 5 Å. Such proximity is consistent with our earlier results21 in which we showed that a replacement of Lys-95(β) with Ile causes a drastic inhibition of gelation. These results emphasize the significant differences between various ionizable surface amino acids in stabilizing the HbS aggregate and suggest that an appreciation of the quantitative contribution of critical amino acid side chains to the aggregation process might show new and more important sites, thus prompting further consideration of developing a therapeutic modality directed at the HbS polymer itself.
ACKNOWLEDGMENT
We thank Adelaide Acquaviva for her expert help with the typescript and Drs Urooj Mirza and Brian Chait for the mass spectrometric analysis.
Supported in part by Grant No. HL-18819 (to J.M.M.) from the National Institutes of Health, Bethesda, MD, and by the Academy of Finland, Helsinki (J.-P.H.).
Address reprint requests to James M. Manning, PhD, Northeastern University, Department of Biology, 360 Huntington Ave, Boston, MA 02115.

![Fig. 6. Gelation concentration (Csat ) of HbS and E6V(β)/D85K(α). Oxy hemoglobin samples in 50 mmol/L potassium phosphate, pH 7.5, were mixed anaerobically with dextran and sodium dithionite, incubated at 37°C, and centrifuged. The hemoglobin concentrations in the supernatant before (initial [Hb]) and after (equilibrium [Hb]) the incubation were determined by amino acid analysis. If the equilibrium [Hb] is lower than the initial [Hb], it represents the gelation concentration of Csat of the Hb.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4196/4/m_bl_0023f6.jpeg?Expires=1764006339&Signature=jm4suW6sMviGGRTg-Cb9tldryG51JwP21voh6aFaIYqtSg62JxI7fraSUUEFipSwO9uG9B6KnBbFw~jx3h6ulP9blIh1fEaIkVbjIXFkCz5pQoMQHKoZOAiGxANwa2uHreX3jwybLZtNXFUO4IQPv~qcaGXy0qSaWeOcF0IU13jG8cAjOwVzqSBBeTh84q4aLeMjNBGNqD0eCxRp8b8SsvDepOOjE7jbPlbOGK3BwHstdrBltJDYGkEPAKJ-lpCuyro9zvIvRrveO1kXw4IYXlQqo7ds9BvtsUoh6WhQEVLDEtvehsTgNWmaKLT5hsqht1mbZg9uAqZobTogUzlJNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. View along the central dyad axis of deoxy HbS. The distances between the two Lys-99 ε-NH2 groups, which is a major chloride-binding site in the center of the axis, and between the oxygen-linked chloride-binding residue (Val-1[α]) and the newly-created mutant residue (Lys-85[α]) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4196/4/m_bl_0023f7.jpeg?Expires=1764006339&Signature=0ZuBamREiwru8yyodp3DxbTcm77xI8CKSm2G9~12MYmtaRwMP9wHGcUiaRGLme16wgW-uZisMZDVVSgDmVWT00kqiF8IE73-2Tth5Rd66lsCR4H265ybVcqj~bsS2Whcgqu-RuE-uuL4DRrkw-bzBo53ayoy35EyELxDh6di8t3Yd80MizsqCTTacwpBvDk1SxvhG0jXH4fERJ2oIrmgFCLbd7FXK96tD7tzloB~8NSsFuNVF0lIfIdv9B4vD-8Qbuc-FDZ9dt6CDL7BsUU7hVCJXQjUscFRljkkwtRiDUXpmbZceC2mNESoQ4H-k6v9gmTzVK8kZnPT3sH1iVFGsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. Gelation concentration (Csat ) of HbS and E6V(β)/D85K(α). Oxy hemoglobin samples in 50 mmol/L potassium phosphate, pH 7.5, were mixed anaerobically with dextran and sodium dithionite, incubated at 37°C, and centrifuged. The hemoglobin concentrations in the supernatant before (initial [Hb]) and after (equilibrium [Hb]) the incubation were determined by amino acid analysis. If the equilibrium [Hb] is lower than the initial [Hb], it represents the gelation concentration of Csat of the Hb.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4196/4/m_bl_0023f6.jpeg?Expires=1764006340&Signature=PsmCt6WPnC0fVFxztA0sJXJFijx6DIb5UZoJxbdodGd8G8ijZsP0pP6RfO3HnhTbPbbMkRsqW8HbmtK6~fGC1Zk1CvRAde8yrICt7LhmShG8V6HT6L020PNpeoKTcswuEn1T-l2xcEEpQOYAXPv68XatCXhIdlTzrbZKDnx5~KCmdIqjsCm1vmLSh6pPz-1XoQ0GDiR5NDLyvGZPTS2HnsFlMU6CM7fO2js4GC775ZDgLbN396jm8~Xeixvq8zZoOomPTdI7Uo3Ne79UlmUIOo10sYKvp0ckbpifQSZAFKvPLcNyBBp1LO-HD-aTzaa7urum~-FIcTFuqJtx4RszYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. View along the central dyad axis of deoxy HbS. The distances between the two Lys-99 ε-NH2 groups, which is a major chloride-binding site in the center of the axis, and between the oxygen-linked chloride-binding residue (Val-1[α]) and the newly-created mutant residue (Lys-85[α]) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4196/4/m_bl_0023f7.jpeg?Expires=1764006340&Signature=32WZZ4Q324gOoD-zKsophPHA-hmMErz22BPbYG2E3jX1160f7pZ7kyPz12f8B4yswjuN5hNSdW8buD~XR58yHcB80VUaI172BL6wo3mL7z3EKw7262gAt0Y~AcHGZTt-1UuI9pqRHz3FVlGXd1W1l3dV22NadW~TWA1F58LCSv~lJa-oL3AftoJQZg0mmUryfo9cLM0y1nuiJKewomOKGwqzoSEJK48T14RrLIMwko~VLsMUecqsW56Wdcfh7iymdJt1cHWDcVGFz~NqvwpxpcmCRhUexCXRWEU62QWIRoRrU3gMRduoKMwdZuSSsz-lgvG1zv8Quw25zn529V2G7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)