Abstract
Follicular lymphoma (FL) is characterized in a significant proportion of cases by the t(14; 18) chromosomal translocation, which results in the juxtaposition of the oncogene bcl-2 to the joining region of the immunoglobulin heavy chain (IgH) gene. Molecular sequence analysis indicates that the t(14; 18) rearrangement occurs in a B-lymphoid progenitor cell at the time of IgH rearrangement. We were interested whether hematopoietic stem and progenitor cells as characterized by CD34 expression bear the translocation. Bone marrow (BM)-CD34+ cells were enriched from 14 patients with FL whose BM was known to be positive for bcl-2/IgH (major breakpoint region [MBR]). Six patients were in complete remission (CR), two patients were in partial remission (PR), and six patients had active disease. Six patients had histological BM involvement when the samples were obtained. Using an immunomagnetic selection device (MINIMACS), a mean purity of 88.7% ± 4% CD34+ cells was achieved. The CD34+ cells were further enriched by fluorescence activated cell sorting (FACS) using CD34 fluorescein isothiocyanate (FITC)- and CD19 phycoerythrin (PE)-conjugated antibodies. The IgH gene was rearranged in the CD34+/CD19+ cell subset of all patients assessed by polymerase chain reaction (PCR). This population is thought to represent the progenitor stage at which the bcl-2/IgH translocation occurs. The unseparated BM mononuclear cell fraction from all 14 patients was positive for bcl-2/IgH using a nested PCR, but the BM-CD34+ cell fraction and the respective CD34+/CD19+ subset were negative in 13 of these 14 patients. The one patient with a positive PCR signal in the CD34+ cell subset had a relapse with BM involvement. We conclude that CD34+ progenitor cells including CD34+/CD19+ B-cell progenitors are not involved in the malignant cell clone. These data are in agreement with a transgenic mouse model, which indicates that the malignant phenotype in FL is sustained by mature B cells.
THE DEVELOPMENT OF mature B cells from a totipotent hematopoietic stem cell is characterized by the formation of a specific Ig receptor. To generate the vast repertoire of antigen-recognizing receptors required throughout life, lymphocytes of mammals have the unique ability to rearrange parts of their DNA in a tightly regulated fashion. The different segments termed as variable (V), diversity (D), and joining (J) regions of the Ig heavy chain gene are flanked by a recombination signal sequence (RSS).1,2 The earliest B-lineage progenitor in man is a CD34+/CD10+/CD19+ cell,3 but there are no data concerning Ig gene rearrangement in particular progenitor cell populations as defined by surface expression of differentiation-associated antigens. In mice, DJ joining occurs at the pre-B–cell stage in the bone marrow in a subset of cells expressing c-kit, B220, and CD43.1
Follicular lymphoma (FL) is characterized in approximately 80% to 90% of cases by a t(14; 18) translocation, which results from the translocation of the bcl-2 gene from band q21 of chromosome 18 to the heavy-chain locus on chromosome 14, band q32.4,5 The DNA region close to the breakpoints on the bcl-2 gene shows sequence similarity to the RSS located at the 5′ end of the D segments of the heavy chain, indicating that the t(14; 18) translocation may occur during VDJ joining in a progenitor cell at the pre-B–cell stage.6 Another hypothesis suggests that one of the segments within the major breakpoint region (MBR) contains an octamer sequence related to χ, the prokaryotic activator of recombination.7 The RAG1 product may therefore interact with the χ-like sequence resulting in the translocation.7 Nearly 70% of the t(14; 18) translocations are located within a 150-bp segment of the bcl-2 gene at the untranslated end of exon 3 in the MBR, whereas approximately 25% of the breakpoints occur in the minor cluster region (mcr) in a segment of 500 bp, localized 20 kb 3′ of the bcl-2 gene.8-10 The translocation results in the transcriptional dysregulation and overexpression of bcl-2, rendering the cells less susceptible to apoptosis-inducing signals.11
We assessed whether CD34+ hematopoietic progenitor and stem cells from patients with t(14; 18)-positive FL contain cells with the t(14; 18) translocation. BM-CD34+ cells from 14 patients were enriched to high purity using a two-step selection technique. The IgH gene was rearranged in the CD34+/CD19+ cell subset of all six patients examined by polymerase chain reaction (PCR), whereas no bcl-2/IgH rearrangement of the MBR was found in 13 of 14 patients whose BM mononuclear cells were t(14; 18) positive.
MATERIALS AND METHODS
Patients.Fourteen patients with follicular non-Hodgkin's lymphoma were scheduled to receive or had undergone granulocyte colony-stimulating factor (G-CSF )-supported cytotoxic chemotherapy for the mobilization of peripheral blood stem cells (PBSC) followed by high-dose therapy with PBSC support. Using a nested PCR, the patients were found to have a t(14; 18) translocation involving the MBR in BM and peripheral blood samples. The patients' characteristics including clinical status, proportion of BM lymphocytes, of CD19+, CD34+ and CD34+/CD19+ cells are detailed in Table 1. The patients were treated in the Departments of Internal Medicine V and Radiology of the University of Heidelberg.
Informed consent was obtained from each patient before they were enrolled onto the study. The cut-off date of this report is August 1, 1996.
Cell preparation.Mononuclear cells (MNC) were separated by Ficoll-Hypaque density centrifugation (density 1.077 g/cm3 ) (Ficoll-Hypaque, Pharmacia, Uppsala, Sweden). The CD34+ fraction was enriched from the BM using an immunomagnetic method (MINIMACS, Miltenyi-Biotec GmbH, Bergisch Gladbach, Germany), according to the manufacturers' instructions. One aliquot of the enriched CD34+ cells was incubated for 30 minutes at 4°C in the presence of an anti-CD34 fluorescein isothiocyanate (FITC)-(HPCA-2) and an anti-CD19 phycoerythrin (PE)-conjugated monoclonal antibody (all from Becton Dickinson, BD, Heidelberg, Germany). Cells were washed twice with phosphate-buffered saline (PBS), and red blood cells were removed using a fluorescence-activated cell sorting (FACS) lysis solution (BD). Immunofluorescence analysis was performed using a five-parameter FACScan (BD) equipped with an argon-ion laser tuned at 488 nm and 0.3 W. Emission from FITC and PE was measured using filters of 530 nm and 585 nm, respectively.
The side scatter characteristics (SSC) versus CD45 fluorescence dot plot were used to discriminate between the smallest hematopoietic cell population and erythrocytes or debris. The CD34+ cells were analyzed in a fluorescence versus SSC plot. Only cells with a lymphoid or lymphomonocytoid appearance were counted as CD34+ cells and their proportion was calculated in relation to that of CD45+ cells. The percentage of false positive events determined using isotype specific control was <0.05% and was subtracted from the percentage of CD34+ cells.
The majority of the CD34+ and CD34− cells isolated by MINIMACS was labelled with an anti-CD34 FITC-, an anti-CD19 PE-conjugated monoclonal antibody or isotype specific controls. CD34+/CD19+, CD34+/CD19−, CD34−/CD19+ and CD34−/CD19− cells were then sorted using a FACSVantage (BD). The side scatter characteristics were also taken into account and only cells with a lymphoblastoid appearance were sorted.
Unless otherwise indicated all results are expressed as mean ± standard error of the mean (SEM).
DNA preparation and PCR amplification.After sorting, DNA was extracted from the different BM subpopulations: MNC, CD34−, CD34−/CD19+, CD34−/CD19−, CD34+, CD34+/CD19+ and CD34+/CD19− cells. DNA from samples containing more than 5 × 104 cells was extracted by cell lysis, proteinase K digestion, and phenol/chloroform extraction. DNA from cells of sorted samples was extracted using a magnetic technique (DNA DIRECT kit, Dynal, Oslo, Norway) according to the manufacturer's instructions. To ensure that amplifiable DNA was present, PCR of the normal bcl-2 gene was performed for 40 cycles, using the primers MBR-1 and MBR-2 (Oncogene Science, Inc, Uniondale, NY). This resulted in the amplification of a 202-bp fragment.5 12
Each DNA sample was then amplified by PCR for the immunoglobulin heavy chain VDJ rearrangement, according to the method of Yamada et al,13 with some minor modifications.14 The following primers were used: 5′-CTCGCTCGCCCATAGAGGAGACGGTGACC-3′ (LJH-CL) and 5′-CTGGTTCGGCCCAACACGGC(C/T)(G/C)TGTATTACTGT-3′ (FR3A-CL). PCR conditions were as follows: 1 minute denaturation at 94°C, 1 minute annealing at 65°C for 15 cycles and at 55°C for 25 cycles, 90 seconds extension at 72°C, with a prolongation of 7 minutes for the final extension step. Samples were electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining.
The samples were amplified for the bcl-2/IgH rearrangement by PCR, using nested oligonucleotide primers, as described,15 16 with some minor modifications. The initial amplification was performed for 40 cycles using the oligonucleotides: 5′-ACCTGAGGAGACGGTGACCAGGGT-3′ for the JH consensus region and 5′-GCAATTCCGCATTTAATTCATGGTATTCAGGAT-3′ for the MBR. Reamplification of a 2-μL aliquot of the PCR product, or of a 1:200 dilution when a specific band was visible on a 2% agarose gel, was performed for 30 cycles using oligonucleotide primers internal to the original primers: 5′- CTCGCTCGCCCAACCAGGGTCCCTTGGCCCCA-3′ and 5′-CTGGTTCGGCCCATTAGAGAGTTGCTTTACGTG-3′. Each cycle consisted of denaturation for 1 minute at 94°C, annealing for 1 minute at 60°C (primary PCR) or 65°C (secondary PCR) and extension for 90 seconds at 72°C. The final extension period was prolonged for 10 minutes.
PCR was performed in a final volume of 50 μL using the DNA extracted from at least 5,000 cells, 40 pmoles of each oligonucleotide primer, 0.2 mmol/L each of deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP), 1 μL of Advantage Klentaq Polymerase Mix in KlenTaq PCR reaction buffer (Clontech Lab, Palo Alto, CA). Samples were electrophoresed on an ethidium bromide-stained 2% agarose gel.
The sensitivity of this PCR technique was studied by mixing 1 t(14; 18)-positive KARPAS-K422 cell17 with various numbers of cells from t(14; 18)-negative normal donors. The length of the bcl-2/IgH PCR product varies between patients as a function of the precise breakpoint position in bcl-2, the presence of IgH D-segments, and modifications of the junctional sequence. A patient-specific positive control was included in every PCR reaction to compare the bcl-2/IgH fragment length with the PCR product obtained at the time of initial diagnosis and to exclude the presence of contaminating DNA. There was also a negative control containing water instead of DNA for each PCR reaction. Each PCR reaction was performed in duplicate, in at least two independent experiments.
RESULTS
Patient characteristics.BM samples from 14 patients with FL were examined. Before the collection of the study samples, all patients had at least one BM and PB sample that was positive for bcl-2/IgH rearrangement involving the MBR. The median number of positive samples (BM or PB) from previous examinations was 5.5 per patient. One patient with active disease was entered onto the study at initial diagnosis, while one patient in relapse was enrolled before and two patients in PR after PBSC mobilization. All four patients had BM involvement as assessed by histological examination. BM samples from 10 patients were obtained during regular follow-up visits after PBSC-supported high-dose therapy. The median time elapsed since high-dose therapy was 21.7 months (range, 8 to 43.5). According to the results of clinical and histopathologic examinations, six of the 10 patients were in CR, whereas four patients were in relapse, of whom two showed histological BM involvement (Table 1).
Immunomagnetic enrichment and FACS-sorting of CD34+ cells and of their CD19 subsets.The mean proportion of BM-CD34+ cells before enrichment was 1.3% ± 0.3% (range, 0.05% to 4.8%), while the mean proportion of these CD34+ cells coexpressing CD19 was 27.4% ± 5.3%. When the gating was limited to CD34+ cells with lymphoblastoid appearance according to SSC, the mean proportion of CD34+/CD19+ cells increased to 34.3% ± 5.4%. Before selection, the BM samples contained a mean proportion of 13.6% ± 1.9% CD19+ cells (Table 1).
After the first enrichment procedure using MINIMACS, the mean purity of CD34+ cells obtained was 88.7% ± 4% with a mean recovery of 69.8% ± 7.6%. At the same time, the proportion of CD19+ cells could be reduced to 0.4% ± 0.1% (range, 0 to 1.4; n = 11).
The FACS-setting used for further purification is shown for one representative patient (K.A.) (Fig 1). From our previous experiments, the mean purity of the CD34+ cells obtained under these conditions was between 99.6% and 99.8%.
Bone marrow-CD34+ and CD34− cells were sorted for their CD19 respective subsets using CD34 FITC- and CD19 PE-conjugated antibodies. To increase the sensitivity of our PCR method at the lymphoid progenitor cell level, only cells with a lymphoblastoid appearance were sorted. The FACS setting of one representative patient (K.A.) is shown. Isotype control for CD34+ cells (A) and for CD34− cells (C) were included. Sorting gates for CD34+ cells (B) and CD34− cells (D) were set to clearly separate between CD19+ and CD19− cells.
Bone marrow-CD34+ and CD34− cells were sorted for their CD19 respective subsets using CD34 FITC- and CD19 PE-conjugated antibodies. To increase the sensitivity of our PCR method at the lymphoid progenitor cell level, only cells with a lymphoblastoid appearance were sorted. The FACS setting of one representative patient (K.A.) is shown. Isotype control for CD34+ cells (A) and for CD34− cells (C) were included. Sorting gates for CD34+ cells (B) and CD34− cells (D) were set to clearly separate between CD19+ and CD19− cells.
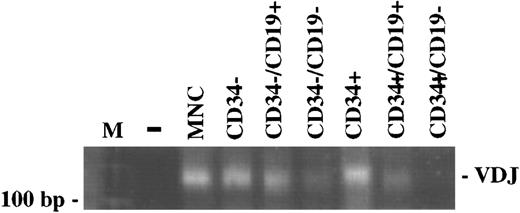
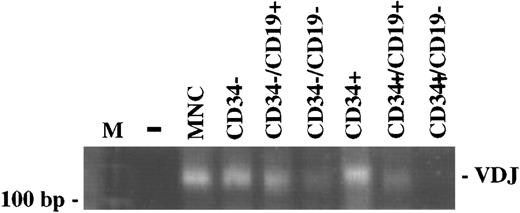
Ig heavy chain gene rearrangement.The status of rearrangement of the Ig heavy chain was assessed in CD34+ cells including the CD19+ B-lymphoid subset, as well as in mature CD19+ cells. Using a PCR method for the detection of VDJ rearrangement, the different subpopulations of BM samples from six patients were examined following FACS-sorting. Three patients in CR and two patients with active disease were examined after PBSCT, one patient in PR was included following PBSC mobilization. The PCR of the unseparated MNC fraction of all patients gave a smear of DNA fragments with a length between 140 bp and 160 bp, indicating polyclonality. The CD34−, CD34−/CD19+, CD34+, CD34+/CD19+ cells were also polyclonal with regard to VDJ rearrangement, while the CD34+/CD19− cells were negative. Figure 2 shows the PCR results from one representative patient (G.M.).
The VDJ rearrangement is detectable in CD34+/CD19+ cells, but not in CD34+/CD19− cells. The PCR of the DNA extracted from the different bone marrow subpopulations separated by FACS sorting, but not of CD34+/CD19− cells, gave a smear of DNA fragments indicating polyclonality. The PCR results from one representative patient (G.M.) are shown. The presence of amplifiable DNA in all samples was confirmed by the amplification of the normal bcl-2 gene (data not shown). (M), 100 bp DNA ladder; (−), negative control; (MNC), mononuclear cells.
The VDJ rearrangement is detectable in CD34+/CD19+ cells, but not in CD34+/CD19− cells. The PCR of the DNA extracted from the different bone marrow subpopulations separated by FACS sorting, but not of CD34+/CD19− cells, gave a smear of DNA fragments indicating polyclonality. The PCR results from one representative patient (G.M.) are shown. The presence of amplifiable DNA in all samples was confirmed by the amplification of the normal bcl-2 gene (data not shown). (M), 100 bp DNA ladder; (−), negative control; (MNC), mononuclear cells.
Assessment of BM-subpopulations for bcl-2/IgH.For the assessment of cells bearing the bcl-2/IgH translocation, a sensitive nested PCR method was used. The size of the amplified products varied between approximately 250 bp and 500 bp after the first round of amplification and between 100 bp and 350 bp after the second round of amplification. The detection limit of this PCR method is 1 t(14; 18)-positive KARPAS-K422 cell17 in 105 to 106 negative MNC (data not shown).
In the 14 patients studied, the unseparated MNC fraction from BM and PB contained bcl-2/IgH–positive cells (Table 1 and Fig 3). The finding was confirmed in at least two samples from both PB and BM. This group of patients included six patients with active disease (one patient at initial diagnosis, one patient with relapse before PBSC mobilization, and four patients with relapse posttransplantation). No significant differences in the amount of DNA input were noted between the different samples from patients assessed by the amplification of the bcl-2 gene.
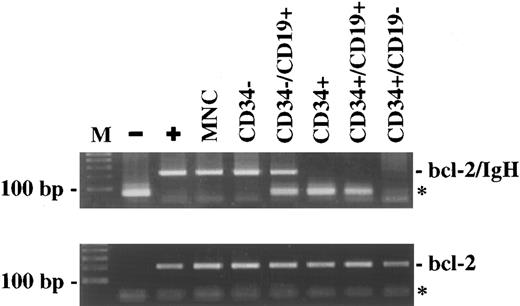
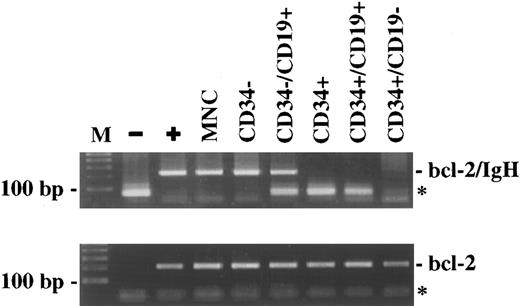
BM-CD34+ cells do not contain the t(14; 18) translocation. CD34+ cells were enriched to high purity using an immunomagnetic technique, followed by FACS sorting for CD34 and CD19 expression. The DNA extracted from the different subpopulations was amplified by a sensitive PCR method for the detection of bcl-2/IgH rearrangement. The PCR results from one representative patient (K.A.) are shown. (M), 100 bp DNA-ladder; (−), negative control; (+), patient-specific positive control; (MNC), mononuclear cells; (*), primer-dimers.
BM-CD34+ cells do not contain the t(14; 18) translocation. CD34+ cells were enriched to high purity using an immunomagnetic technique, followed by FACS sorting for CD34 and CD19 expression. The DNA extracted from the different subpopulations was amplified by a sensitive PCR method for the detection of bcl-2/IgH rearrangement. The PCR results from one representative patient (K.A.) are shown. (M), 100 bp DNA-ladder; (−), negative control; (+), patient-specific positive control; (MNC), mononuclear cells; (*), primer-dimers.
After MINIMACS-selection and FACS-sorting, the CD34− cells and the CD34−/CD19+ cells were also bcl-2/IgH–positive (Table 1), whereas the CD34−/CD19− cells were bcl-2/IgH–negative in four of seven patients examined. Looking at the progenitor and stem cell compartments, the CD34+ cells, including the subset of CD34+/CD19+ cells, were PCR-negative in 13 cases (Table 1). Figure 3 shows the PCR results of one representative patient (K.A.). On the other hand, there was only one patient with a positive PCR signal in the CD34+ and CD34+/CD19+ cell population. PCR positivity was confirmed in two samples obtained at two different follow-up visits 39.5 and 43.5 months after PBSCT when the patient was in relapse involving lymphnodes and BM with an infiltration of approximately 25%. The CD34+/CD19− cells were negative for the t(14; 18) translocation in this patient, as in all the other 13 cases.
DISCUSSION
High-dose chemotherapy with the support of enriched CD34+ progenitor and stem cells is currently used to treat patients with a variety of hematological malignancies and solid tumors. Ex vivo enrichment is theoretically beneficial only if the malignant cells and their progenitors lack CD34 expression. This report shows that, in 13 of 14 patients with FL and t(14; 18)-positive mononuclear BM cells, CD34+ cells from BM, including their respective CD19+ subset, do not bear the t(14; 18) translocation. From our findings, one may conclude that CD34+ cells from patients with a t(14; 18)-positive FL do either genuinely not bear the translocation or their involvement is below the detection limit of our method.
To reduce the possibility of contamination by more mature CD19+ malignant cells lacking CD34 expression, a two-step CD34 selection procedure was used, combining a CD34+ cell immunomagnetic selection with two-color FACS sorting for CD34 and CD19. The majority of our patients presented with a normal CD19+ cell count in BM. One of the patients was examined at the time of initial diagnosis, and five patients were in relapse. Because the CD34+ fraction of five patients with active disease was bcl-2/IgH–negative, we assume that the malignant precursor cell of FL must be a cell of more mature phenotype. The only patient with a positive PCR-signal in the CD34+ and CD34+/CD19+ cell fraction was a patient in relapse after PBSCT presenting with a BM infiltration of approximately 25%. In this case, contamination of the CD34+ and CD34+/CD19+ cell fraction by mature CD19+ cells may account for the PCR positivity observed.
The results of our study are at variance with recent findings of Macintyre et al,18 who showed positivity for bcl-2/IgH (MBR) in the FACS-sorted CD34+ cell population of five patients with FL. FACS-sorting was performed using BM samples without an immunomagnetic CD34+ cell preselection, resulting in a purity of CD34+ cells greater than 95%. Besides the different enrichment and PCR method used, the apparent discrepancy may also have resulted from differences between the patient populations examined. In contrast to our patient group, the patients in their study had a large tumor burden with significant BM infiltration with abnormal lymphocytes in a proportion between 11% and 45%. In agreement with our findings, the CD34+/CD19− cell fraction was PCR-negative in four of five patients.18
Gorin et al19 obtained data similar to ours in that CD34+ cells enriched by large scale-immunoaffinity column were PCR-negative in eight of nine BM harvests from eight patients with t(14; 18)-positive FL. The median purity of the CD34+ cell fraction was 49% (range, 12% to 80%), whereas the median proportion of CD34+/CD19+ lymphoid progenitors was 11% (range, 1% to 69%). The BM from these patients was rated negative by conventional cytology and histopathology.19
The t(14; 18) translocation is thought to occur at the time of IgH rearrangement.6 At a later stage of differentiation, secondary genomic alterations may give rise to the malignant phenotype in a precursor cell lacking CD34 expression. This could be concluded from our findings showing that patients with t(14; 18)-positive B cells lack bcl-2/IgH rearrangement in their CD34+/CD19+ progenitor cells following VDJ assembly, which represents the critical period for the translocation. Similarly, bcl-2/IgH transgenic mice show extended survival of B cells, but progress from polyclonal to monoclonal disease and develop malignant lymphoma only after a variable period of latency and secondary transforming events.20,21 The ambiguous nature of a positive PCR finding for t(14; 18)-bearing cells as a potentially premalignant stage has been further supported by several groups showing bcl-2/IgH–positive cells in PB samples, and particularly in B lymphocytes of 40% to 50% of normal donors.22-24 Similarly, Poetsch et al25 reported the detection of small populations with t(14; 18) in 12.5% of nonneoplastic lymphoproliferations by fluorescent in situ hybridization (FISH). In addition to the t(14; 18) translocation, a number of adjunctive chromosomal abnormalities have been described in FL, and comparative genomic hybridization (CGH) showed complex genetic changes.26 Class-switch, somatic hypermutation in the Ig genes, and clonal selection of the tumor cells of patients with FL also reflect further changes in the malignant cells as the disease evolves.27 28
In light of these findings and the results of our study, we hypothesize that a nonmalignant t(14; 18)-positive CD34+ progenitor cell migrates from the BM to lymph nodes, where additional genomic events may occur at a later stage of B-cell differentiation leading to the neoplastic precursor cell lacking CD34 expression. Extending this line of thought, BM or organ involvement by t(14; 18)-positive cells is rather the result of hematogenous spread and infiltration by more mature malignant B cells with sustained self-renewal capacity.
ACKNOWLEDGMENT
We gratefully acknowledge R. Schulze and K. Kiel for technical suggestions, M. Pförsich, L. Volk, and K. Hexel (Department of Tumor Immunology, German Cancer Research Center, Heidelberg, Germany) for the excellent technical assistance with FACS analysis and FACS sorting. We further acknowledge M. Deichman for helpful suggestions, D. Maclachlan for comments on the manuscript, and U. Scheidler for expert secretarial assistance.
Address reprint requests to Maria Teresa Voso, MD, Department of Internal Medicine V, University of Heidelberg, Hospitalstr. 3, 69115 Heidelberg, Germany.