Abstract
We evaluated 56 consecutive patients with newly diagnosed lymphoma including 48 with non-Hodgkin's lymphoma (NHL) and 8 with Hodgkin's disease to determine the clinical and prognostic significance of magnetic resonance imaging (MRI) of the femoral marrow. MR images of the femoral marrow were obtained by the T1-weighted spin echo method and the short TI inversion recovery technique. Abnormal “positive” images were seen in 29 of the 56 patients (52%). All 17 patients with positive biopsy results showed abnormal images on their femoral marrow MRI. Three “positive” MRI patterns — scattered (72%), uniform (21%), and nodular (7%) — were observed. The overall survival of the patients with a positive MRI pattern was significantly poorer than that of patients with a normal pattern (P = .0129). Survival did not differ significantly according to MRI pattern. The 3-year survival rate in the patients with a normal MRI pattern was 89.9% and in the patients with a positive MRI pattern, it was 41.0%. This difference was statistically significant (P = .0279) when we evaluated only the patients with NHL. Patients with positive MRI patterns, but a normal bone marrow histology, showed a significantly shorter survival than those with a normal MRI pattern (P = .016). These results indicate that abnormal MR images of the femoral marrow are associated with a significantly poorer survival in patients with malignant lymphoma, regardless of histologic findings in the marrow.
THE DETECTION of the bone marrow (BM) involvement is essential in the clinical staging and treatment of patients with malignant lymphomas. While evidence of such involvement is usually obtained by blind BM biopsies and aspirates, these minute samples may fail to provide an adequate representation of the BM. Malignant lymphomas are characterized by a heterogeneous pattern of marrow involvement. The incidence of marrow involvement in non-Hodgkin's lymphoma (NHL) is highest in patients with small lymphocytic lymphoma (>80%) and mantle cell NHL.1-5 More than 50% of the cases of follicular NHL exhibit such involvement.1,4 In diffuse aggressive lymphoma, the incidence of involvement of the marrow ranges from 5% to 34%,2,6 and 4% to 14% of patients with Hodgkin's disease have BM involvement.7
Because magnetic resonance imaging (MRI) presents a more global view of the BM than does biopsy material, such imaging may provide a better understanding of disease progression and remission.8-13 MRI also allows one to assess the entire marrow compartment, providing information about regions that cannot be sampled by biopsy or aspiration. The MRI is essentially free of bone interference and is superior to other methods in terms of contrast resolution for fat and cellular components. In T1-weighted spin echo (SE) images, the fatty marrow appears bright, whereas the cellular marrow, with its lower fat content, exhibits a low signal intensity. Short TI inversion recovery (STIR) sequence images are sensitive to a prolongation of T1 and T2 relaxation times and are designed to suppress the signal from the surrounding fatty tissues.14 The femoral marrow of adults is largely fatty.13 The femur allows for evaluation of malignant involvement and assessment of disease severity over time using a single bone.12 MRI of the femoral marrow, therefore, seems to be the most suitable method for detecting involvement of the marrow in adults with hematologic malignancies. Results of MRI of the marrow in patients with lymphoma have been reported in several small series, and suggest that involvement of the BM may be detected by MR imaging, and that results of MRI may complement those obtained by biopsy.15-21 We report the femoral marrow MRI findings in a consecutive series of untreated patients with lymphomas, and discuss the MRI patterns, their correlations with clinical findings, and their influence on prognosis.
MATERIALS AND METHODS
Patients.We studied retrospectively the data on 56 untreated Japanese patients (33 men and 23 women, aged 13 to 86 years, median, 58 years) with lymphomas who visited the Omiya Medical Center at Jichi Medical School, Omiya, Japan, between March 1990 and March 1995. Of 56 patients, 48 had NHL and 8 had Hodgkin's disease. Lymphomas were classified as non-Hodgkin's lymphoma or Hodgkin's disease and subclassified according to the Working Formulation.22 Patients with adult T-cell leukemia/lymphoma were excluded from this study. The clinical features of the patients are listed in Table 1. MRI was performed at the time of diagnosis in all patients. BM biopsy was obtained from the posterior iliac crest at the time of diagnosis. Bilateral BM biopsy was performed in 20 patients. Unilateral BM biopsy and BM aspiration were performed in 36 patients. In all 17 patients with positive BM biopsy result, the diagnosis of marrow involvement was made by unilateral BM biopsy and BM aspiration. Serum levels of lactate dehydrogenase (LDH) and hemoglobin levels were evaluated at the time of MRI. Overall survival was calculated from the time of MRI evaluation. Disease stage was evaluated according to the Ann Arbor system.23 Patients with NHL were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or equivalent chemotherapy. Patients with Hodgkin's disease were treated with MOPP-ABVD (nitrogen mustard, vincristine, procarbazine, prednisolone-doxorubicin, bleomycin, vinblastine, dacarbazine).
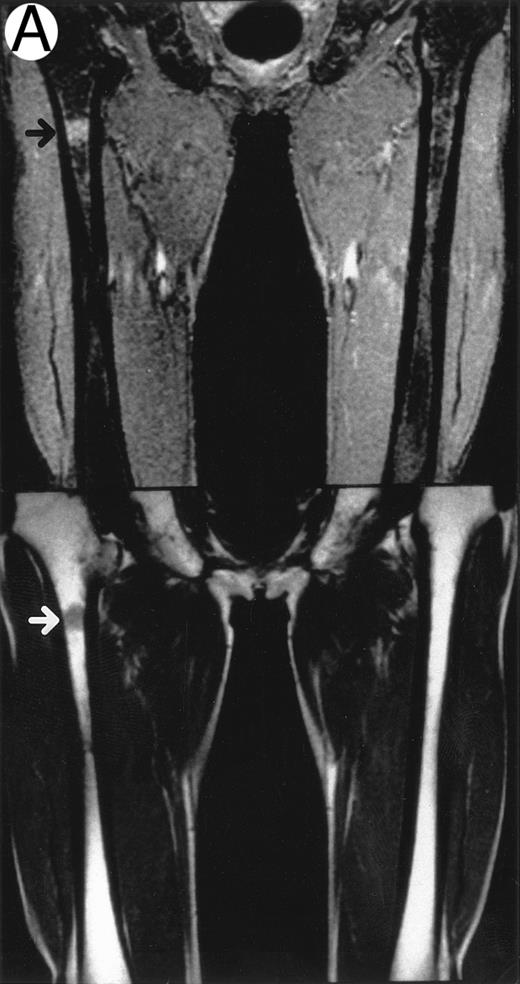
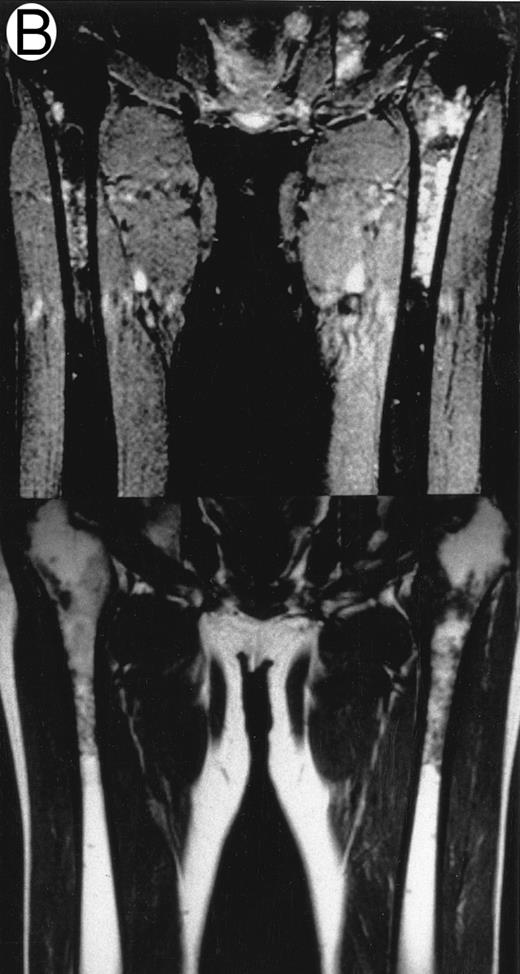
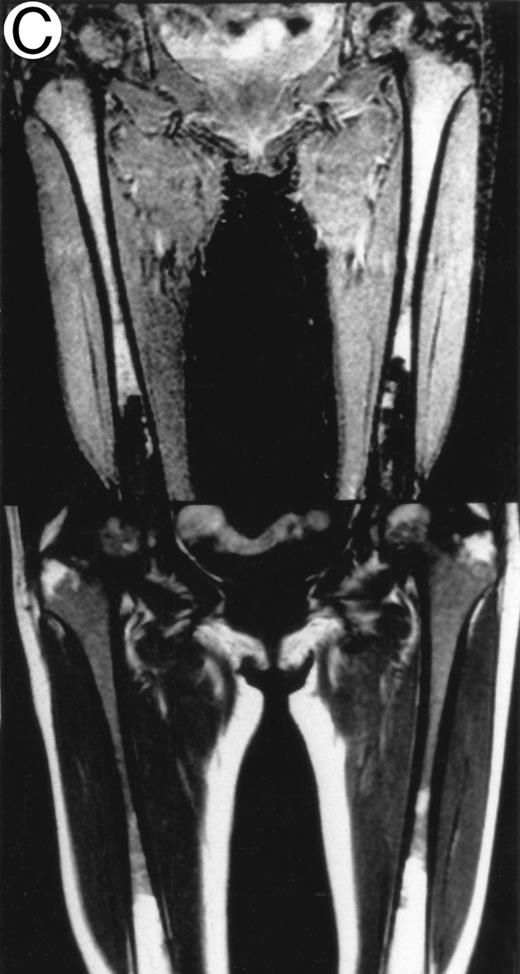
MRI.MRI was performed using SE sequences with the whole-body coil in a 1.5 tesla superconducting system (MRT 200FX/II; Toshiba, Tokyo, Japan). Coronal T1-weighted SE images of the femur were obtained in contiguous 10-mm slices in a 256 × 256 matrix with TR, 400 ms; TE, 20 ms; and number of signal acquisitions, 2. Tissues with short proton T1, such as fatty tissue, have a high signal intensity and appear bright on T1-weighted SE images, whereas those with a long T1, such as cellular marrow, have a low signal intensity and appear dark. STIR coronal images of the femur were obtained in 10-mm slices in a 256 × 256 matrix with TR, 1,500 ms; TE, 20 ms; and TI, 150 ms. On STIR images, the signal from fatty tissue is eliminated, whereas the signal from tissues with a longer T1 is progressively brighter. The MRI results were evaluated blindly by two independent observers who had no knowledge of the patients' marrow histology, tumor type, or stage. The MRI was classified as positive when both observers classified the T1-weighted SE images and STIR images as abnormal. The positive findings on femoral marrow MRI were categorized as follows: nodular pattern, characterized by nodular areas of fatty marrow replacement with a signal intensity that was lower on T1-weighted SE images (higher on STIR images) than that of muscles; scattered pattern, characterized by multiple scattered foci of marrow replacement on a background of uninvolved marrow; and uniform pattern, characterized by a uniform replacement of fatty marrow (Fig 1).
Patterns observed on femoral marrow MRI. (A) Nodular (arrow), (B) scattered, and (C) uniform. Top: STIR images and, bottom: T1-weighted SE images.
Patterns observed on femoral marrow MRI. (A) Nodular (arrow), (B) scattered, and (C) uniform. Top: STIR images and, bottom: T1-weighted SE images.
Statistical analysis.Data are reported as mean ± standard deviation (SD). Statistical analysis of MRI patterns and the clinical parameters used one-way factorial analysis of variance (ANOVA). Fisher's protected least significant difference (PLSD), Scheffe's F, and Bonferroni/Dunn multiple comparison test were used as post hoc tests. Statistical analysis of MRI patterns and the International Prognostic Index used two-way ANOVA. Curves of overall survival relating to the MRI patterns were constructed using the method of Kaplan and Meier.24 Statistical significance was determined by the log-rank test.25
RESULTS
Bone marrow involvement and MRI of femoral marrow.Of the 56 newly diagnosed patients with malignant lymphomas, 29 (51.8%) had abnormal positive MRIs of the femoral marrow on both T1-weighted SE and STIR images (Table 2). Seventeen patients (30.4%) had positive findings on both the MRI and biopsy. All patients with positive biopsy results showed abnormal images on their femoral marrow MRI. Twelve patients (21.4%) showed positive findings on MRI of the femoral marrow despite a normal BM histology. Follow-up examination was performed in 11 of these 12 patients. The abnormalities in the femoral marrow MRI of 5 patients resolved following a successful treatment. The involvement of the BM was confirmed in 4 of 12 patients (by further BM biopsy or aspiration in 3 patients, and by the presence of lymphoma cells in the peripheral blood in 1 patient). One other patient whose BM remained abnormal on MRI exhibited pancytopenia with no apparent involvement of BM observed on biopsy. One patient could not confirm BM involvement by the follow-up examination. One remaining patient died soon after MRI and could not be evaluated.
MRI patterns of femoral marrow.MRI findings of the femoral marrow were classified into three patterns as shown in Fig 1. Of 29 patients with a positive MRI, 21 (72%) exhibited a scattered pattern (Fig 1A), 6 patients (21%) demonstrated a uniform pattern (Fig 1B), and 2 patients (7%) exhibited a nodular pattern (Fig 1C). Of the 17 patients with positive results on BM biopsy, 11 demonstrated a scattered pattern on the MRI, and 6 patients showed a uniform pattern.
Histology of lymphoma and femoral marrow MRI.As shown in Table 3, patients were analyzed in relation to pathologic diagnosis. None of the patients with low grade lymphoma showed normal findings on MRI despite positive biopsy results. Negative BM biopsy results despite positive MR imagings were observed in 12 patients with various lymphomas (1 patient [10%] with low-grade NHL, 9 patients [28%] with intermediate NHL, 1 patient [20%] with high-grade NHL, and 1 patient [13%] with Hodgkin's disease). There was no correlation between histologic findings and the particular pattern observed on the femoral marrow MRI.
Immunophenotype and femoral marrow MRI.The frequency of positive MRI/negative BM biopsy patients was similar in the patients with T-cell and B-cell lymphomas (25% v 21%), while the frequency of patients who had positive findings on both the MRI and biopsy was slightly higher in patients with T-cell lymphomas (50%) than in those with B-cell lymphomas (32%). Interestingly, a uniform MRI pattern was observed only in the patients with B-cell lymphomas.
Clinical parameters and MRI pattern of femoral marrow.If an abnormal signal intensity on the femoral marrow MRI reflects the lymphomatous volume in the bone marrow, biochemical indices such as serum lactate dehydrogenase (LDH) may correlate with the MRI pattern. It is suggested that, of the three abnormal patterns, the uniform pattern may reflect the highest tumor burden. The serum LDH levels in the patients with a uniform pattern, however, did not differ significantly from those in patients with other MRI patterns. We also investigated the influence of the hemoglobin level obtained at the time of the MRI evaluation on the marrow MRI pattern. The hemoglobin value in patients with a normal, nodular, scattered, or uniform pattern was 12.4 ± 2.5, 12.1 ± 0.4, 11.5 ± 2.4, and 9.6 ± 1.4 g/dL (mean ± SD), respectively, with no significant differences observed between groups.
International Prognostic Index and femoral marrow MRI.An International Prognostic Index has been proposed for use in predicting the long-term survival of patients with aggressive NHL.26-29 In the present study, 47 patients with NHL were classified according to this index before MRI examination. The high-risk group as defined by this index included four of six patients (67%) who showed a uniform pattern on their femoral marrow MRI, and 10 of 19 patients (53%) with a scattered pattern. Only 2 of 17 (12%) high-risk patients showed negative results on marrow MRI, whereas 11 of 16 (69%) low-risk patients had negative results on marrow MRI. Thus, patients with abnormal signal intensity on the femoral marrow MRI may have a poor prognosis, although the MRI patterns among these four risk groups did not differ significantly (P > .1).
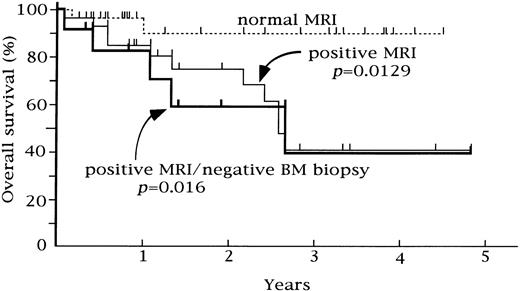
Relationship between overall survival and femoral marrow MRI in 56 patients. Overall survival was significantly poorer in the patients with a positive MRI and patients with positive MRI/normal BM biopsy (P = .0129 and P = .016, respectively).
Relationship between overall survival and femoral marrow MRI in 56 patients. Overall survival was significantly poorer in the patients with a positive MRI and patients with positive MRI/normal BM biopsy (P = .0129 and P = .016, respectively).
Overall survival and femoral marrow MRI.Patients were followed for 1 to 58 months after their MRI evaluation (median, 17 months). As shown in Fig 2, patients with a normal pattern on the femoral marrow MRI had a significantly longer overall survival than those with abnormal patterns (P = .0129). For the group with a normal MRI, the overall survival rate was 89.9% at 3 years, while that of patients with a positive MRI pattern was 41.0% at 3 years. The difference was statistically significant (P = .0279) when we evaluated only the patients with NHL. Patients who exhibited abnormalities of the femoral marrow on MRI, despite a normal BM histology, showed a significantly poorer survival than those with a normal MRI pattern (P = .016). The overall survival rate did not differ significantly according to the pattern of MRI abnormality.
DISCUSSION
The present results indicate that MRI of the femoral marrow may be a sensitive way of detecting lymphomatous involvement of the marrow in untreated patients with malignant lymphomas, and that abnormalities on the femoral marrow MRI may indicate a poor prognosis. Femoral marrow MRIs detected occult involvement of the marrow in 29 of the 56 patients (52%) with (17 patients) or without (12 patients) a positive marrow biopsy. Several investigators have reported results of comparisons of MR images of BM in patients with malignant lymphomas.15-20 In these early reports, abnormal signal intensity was detected on marrow MRI in 18% to 43% of patients with malignant lymphomas. In the present study, no patient with marrow involvement detected by BM biopsy showed a normal MRI of the femoral marrow. Marrow involvement is often missed on MRI in patients with low-grade NHL.15 17 The present results suggest that femoral marrow MRI may be particularly advantageous for detection of marrow involvement in patients with low-grade NHL.
The most important observation of the present study was that an abnormal MR imaging on femoral marrow was significantly associated with a poorer overall survival in patients with malignant lymphoma. Even those patients with negative biopsy results, but positive marrow MRI findings, demonstrated a poor survival. This poor survival of patients with abnormal MRI images strongly suggests occult involvement of marrow by lymphoma cells. The observation of resolution or progression of abnormal signal intensity on follow-up MRI may provide indirect evidence of lymphomatous marrow involvement in these patients. Further BM biopsies may increase the yield of positive BM biopsies. MR images of additional areas of the marrow may also increase the probability of a correct diagnosis, although the present study is limited to femoral marrow. The presence of lymphoma cells in the peripheral blood indicates marrow involvement. Hoane et al15 have reported that the causes of abnormal signal intensity on marrow MRI were confirmed in 60% of patients with positive MRI findings despite a normal BM histology, after such follow-up. In the present study, follow-up examinations confirmed that an abnormal MRI of the femoral marrow may represent occult marrow involvement in 75% of these patients. When it is difficult to confirm involvement of the BM, careful clinical observation is required.
The patients with untreated lymphomas in the present study showed nodular, scattered, or uniform MRI patterns. If a nodular pattern implies a limited involvement of the BM by lymphoma cells and, conversely, if a uniform pattern indicates a diffuse involvement of the marrow, the frequency of the lymphomatous involvement of the marrow detected by BM biopsy from the posterior iliac crest should be correlated with these MRI patterns. In the present study, the frequency of marrow involvement detected by BM biopsy in patients with nodular, scattered, and uniform patterns was 0%, 52%, and 100%, respectively. If these MRI patterns of femoral marrow do, indeed, represent lymphomatous involvement of the marrow, there may be some correlation between the distribution or extent of marrow involvement and the character (ie, pathology and immunophenotype) of the lymphoma cells. In the present study, neither the pathology of lymphomas nor the immunophenotype influenced the MRI pattern. Interestingly, all patients in our study who showed a uniform pattern on the MRI had B-cell lymphomas. Further study is needed to determine whether B-cell lymphomas involves the BM more uniformly than do T-cell lymphomas.
Marrow involvement in patients with lymphomas provides evidence of advanced disease. Clinical parameters such as the level of LDH, the number of extranodal sites of disease, and tumor size also reflect disease progression.28 However, in the present study, the serum LDH level failed to show a significant correlation with the MRI patterns.
An abnormal signal intensity on the femoral marrow MRI may be influenced by physiological factors such as age-related changes in marrow distribution, and by pathological status such as anemia or the presence of other space-occupying marrow diseases.8 29-32 In the present study, the median age of the 56 patients was 58 years, and only three patients were under the age of 20. An age-related conversion of the marrow from red to yellow did not seem to influence our MRI results. Our patients had no other diseases of the marrow that could have influenced the MRI. In addition, the hemoglobin level at the time of MRI evaluation was unrelated to the MRI pattern.
In conclusion, the present results indicate that MRI of the femoral marrow may be a sensitive method of detecting marrow involvement that is not shown by BM biopsy. An abnormal MRI of the femoral marrow appeared to predict a poorer overall survival. MRI of the femoral marrow may allow for a more accurate staging and a more appropriate treatment of patients with lymphoma.
Address reprint requests to Shojiro Takagi, MD, Division of Hematology, Omiya Medical Center, Jichi Medical School, 1-847 Amanuma-cho, Omiya, Saitama 330, Japan.