In this issue of Blood, Zhao et al1 uncover a critical role for mechanistic target of rapamycin complex 2 (mTORC2)/forkhead box protein O1 (Foxo1) as iron sensors in liver sinusoidal endothelial cells (LSECs). They demonstrated that iron triggers lysosomal degradation of the Rictor subunit of the mTORC2 complex, which allows Foxo1 to translocate to the nucleus for transcriptional activation of bone morphogenetic protein 2 (BMP2) and BMP6.
BMP2 and BMP6 are major inducers of the iron regulatory hormone hepcidin. Secreted by LSECs in response to iron, they act as homo- or heterodimeric ligands that bind to BMP receptors on hepatocytes. This interaction activates the Smad signaling cascade, ultimately leading to transcriptional induction of the Hamp gene that encodes hepcidin. Under conditions of elevated iron, hepcidin limits further iron entry into the circulation by inactivating the iron exporter ferroportin on duodenal enterocytes, tissue macrophages, and other cell types. In this homeostatic feedback loop, hepcidin binds to and occludes ferroportin’s iron export channel and triggers ferroportin’s internalization and degradation, thereby blocking dietary iron absorption and iron export from erythrophagocytic macrophages to prevent systemic iron overload.
Although progress has been made, how LSECs sense and respond to iron remains incompletely understood. Genetic and biochemical experiments revealed several upstream contributors, including the uptake of circulating transferrin-bound or non–transferrin-bound iron (NTBI)2,3 and iron retention mediated by paracrine inhibition of LSEC ferroportin by hepcidin secreted from hepatocytes.4 In mice with chronic iron overload, the expression of BMP6, which is more responsive to iron than BMP2, was shown to be transcriptionally activated via the redox-sensitive transcription factor Nrf2.5 However, Nrf2-deficient LSECs retain substantial BMP6-inducing capacity. Thus, further candidates have been proposed to be involved in iron sensing, such as the transcription factors Ets1 or c-Jun and the p38/JNK MAP pathway.6,7
Zhao et al examined the possible role of Foxo1 as LSEC iron sensor. This transcription factor belongs to the Forkhead box O protein family and is well characterized for its function as a master regulator of hepatic glucose and lipid metabolism that responds to nutrient availability, and as a regulator of endothelial cell proliferation or quiescence.8 Recent work demonstrated that Foxo1 also responds to iron and promotes the transcriptional induction of hepcidin through the BMP/Smad signaling in hepatocytes.9 This mechanism involves Foxo1 binding to evolutionary conserved Foxo1-response elements located near established Smad-binding sites within the Hamp promoter.
By using cell culture and mouse models of acute and chronic iron loading, Zhao et al showed that transferrin-bound iron or NTBI promote the nuclear translocation of Foxo1 in LSECs. They explored the physiological relevance of this finding in tamoxifen-inducible, endothelial cell–specific Foxo1 knockout mice. Upon Foxo1 ablation at the age of 3 weeks, the animals developed an iron overload phenotype that was characterized by low expression of hepcidin, BMP2, and BMP6, high serum iron and transferrin saturation, iron accumulation in liver parenchymal cells, and reduced splenic iron content. These responses, which were exacerbated when the mice were fed an iron-enriched diet, are the hallmarks of hemochromatosis, a genetically heterogenous endocrine disorder of iron overload cause by hepcidin insufficiency.10
Biochemical studies showed that the selective Foxo1 inhibitor AS1842856 attenuated iron–mediated induction of BMP2 and BMP6 in primary LSEC cultures. Conversely, overexpression of a constitutively active Foxo1 mutant or pharmacological treatment with the selective Foxo1 activator LOM612, significantly enhanced BMP2 and BMP6 upregulation in response to iron. Prompted by these findings, Zhao et al identified 3 putative Foxo1-binding sites within the promoters of the Bmp2 and Bmp6 genes, which were validated by chromatin immunoprecipitation–quantitative polymerase chain reaction and luciferase reporter assays.
In another set of biochemical experiments, Zhao et al showed that iron loading (acute or chronic) suppresses mTORC2 activity in LSECs (but not in hepatocytes) via lysosomal degradation of its core subunit Rictor. Pharmacologic inhibition of mTORC2, but not mTORC1, significantly increased the expression of Bmp2 and Bmp6, thereby establishing mTORC2 as a negative regulator of these genes. The suppression of mTORC2 led to reduced phosphorylation of the downstream ABC kinases Akt, PKCα, and SGK1. Among them, Akt is known to inhibit Foxo1 by promoting its nuclear export via phosphorylation. Selective pharmacologic inhibition of Akt, but not the other AGC kinases, recapitulated the iron-induced upregulation of Bmp2 and Bmp6, whereas constitutively active Akt abrogated this response. Furthermore, Foxo1 inhibition with AS1842856 reversed the elevated Bmp2 and Bmp6 expression in Rictor-deficient LSECs. Collectively, these data indicate that mTORC2-Akt-Foxo1 signaling is an iron-sensitive regulatory axis in LSECs that governs the transcriptional regulation of BMP2 and BMP6.
Along these lines, mice with tamoxifen-inducible endothelial cell–specific Rictor disruption at the age of 8 weeks, developed a phenotype of iron restriction, which was characterized by the upregulation of hepcidin, BMP2 and BMP6, low serum iron and transferrin saturation, reduced liver iron content, and iron retention in the spleen. The above data highlight the translational potential of Foxo1 activation in the context of iron overload disorders. Zhao et al explored this by developing a lipid nanoparticle–encapsulated plasmid that encoded constitutively active Foxo1 under control of the endothelial cell–specific Cdh5 promoter. Delivery of this construct to Hfe-/- mice, a model of hemochromatosis, increased the expression of BMP2, BMP6, and hepcidin and eventually ameliorated systemic iron overload in these animals over a period of 4 weeks.
Overall, the study by Zhao et al offers important new insights into the mechanism through which LSECs sense iron to regulate the expression of BMP2 and BMP6 (see figure) and their downstream target hepcidin, thereby contributing to systemic iron homeostasis. At the same time, it raises several new questions that warrant further investigation. For example, it is unclear how the mTORC2-Akt-Foxo1 axis integrates with other previously identified iron-sensing pathways.3,5-7 Although Zhao et al presented preliminary evidence that Foxo1 and Nrf2 exhibit distinct temporal responses to iron, the broader regulatory network has yet to be fully uncovered. In addition, the apparent restriction of Foxo1 regulation by the mTORC2-Akt pathway in LSECs but not hepatocytes raises questions about the factors that underlie this cell-type specificity. Finally, the molecular mechanism through which iron triggers lysosomal degradation of Rictor remains elusive. Deciphering this process may be the key to understanding how Foxo1 decodes iron signals in the liver endothelium.
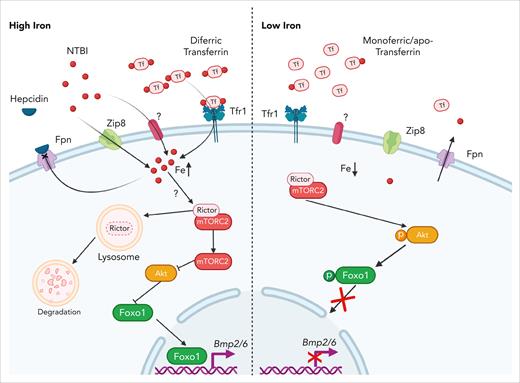
A model for iron sensing in LSECs. High intracellular iron levels, a consequence of the uptake of diferric transferrin via transferrin receptor 1 (Tfr1), NTBI via Zip8 or other transporter(s), or reduced iron efflux caused by hepcidin-mediated inhibition of ferroportin, promote lysosomal degradation of Rictor, a core component of mTORC2. Loss of mTORC2 activity leads to Akt inactivation, thereby enabling the nuclear translocation of Foxo1. Once in the nucleus, Foxo1 binds specific elements in the Bmp2 and Bmp6 promoters, thereby activating their transcription. Conversely, when intracellular iron levels are low because of reduced uptake and/or increased efflux, mTORC2 remains active, phosphorylating Akt. Activated Akt phosphorylates Foxo1, retaining it in the cytoplasm and thereby repressing Bmp2 and Bmp6 expression. Fpn, ferroportin; Tf, transferrin. Figure created with BioRender.com.
A model for iron sensing in LSECs. High intracellular iron levels, a consequence of the uptake of diferric transferrin via transferrin receptor 1 (Tfr1), NTBI via Zip8 or other transporter(s), or reduced iron efflux caused by hepcidin-mediated inhibition of ferroportin, promote lysosomal degradation of Rictor, a core component of mTORC2. Loss of mTORC2 activity leads to Akt inactivation, thereby enabling the nuclear translocation of Foxo1. Once in the nucleus, Foxo1 binds specific elements in the Bmp2 and Bmp6 promoters, thereby activating their transcription. Conversely, when intracellular iron levels are low because of reduced uptake and/or increased efflux, mTORC2 remains active, phosphorylating Akt. Activated Akt phosphorylates Foxo1, retaining it in the cytoplasm and thereby repressing Bmp2 and Bmp6 expression. Fpn, ferroportin; Tf, transferrin. Figure created with BioRender.com.
Conflict-of-interest disclosure: The author declares no competing financial interests.