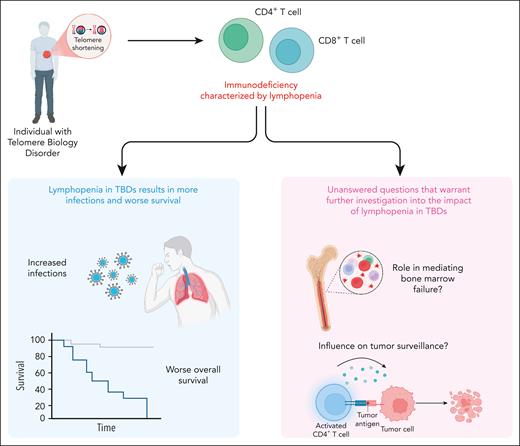
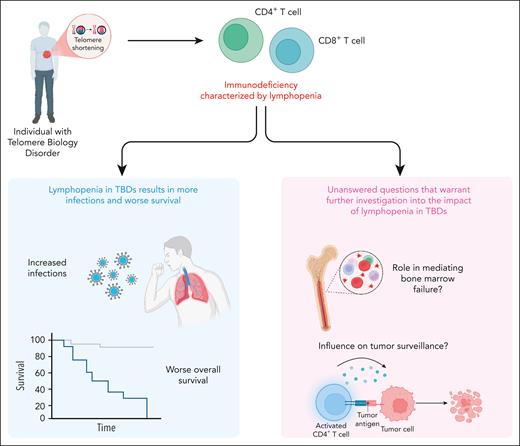
In this issue of Blood, Bazzo Catto et al1 present a detailed retrospective analysis of 88 predominantly adult patients with telomere biology disorders (TBDs) and uncovered a broad spectrum of immune abnormalities caused by T-cell lymphopenia. They demonstrated that the decreased absolute lymphocyte count (ALC) correlated with bone marrow failure, increased infection risk, and worse survival, including the association of low T-cell counts with the development of solid tumors.
TBDs are a spectrum of inherited conditions caused by germ line mutations in genes essential for telomere maintenance, such as TERT and TERC.2 These disorders lead to progressive telomere shortening, which leads to multisystem manifestations including bone marrow failure, pulmonary fibrosis, hepatic disease, and an elevated risk for malignancy. Immunodeficiency is a core feature of TBDs, particularly in the more severe phenotypes that present at a younger age, such as those with dyskeratosis congenita (eg, DKC1).3 It is increasingly recognized in adult-onset forms in which the immunodeficiency may be subtle but clinically significant.4,5 Understanding the nature and extent of immune compromise across the spectrum of TBDs is therefore critical to improving risk stratification, clinical monitoring, and personalized therapeutic strategies.
The study by Bazzo Catto et al highlights that individuals with mostly TERT or TERC germ line mutations, who often present in adolescence or adulthood with milder phenotypes,2 can have significant immunologic abnormalities. A novel finding of this study is that in adults with TBDs, the immunodeficiency is characterized by lymphopenia, not neutropenia or hypogammaglobulinemia that is more typically observed in pediatric TBD.3,6 The divergence between adult and pediatric patients with TBDs underscores the probable importance of implementing age- and genotype-tailored immune monitoring strategies. These data suggest that applying a pediatric-focused screening approach to adults may miss clinically significant T-cell defects that confer an increased risk for infection, bone marrow failure, or malignancy. Conversely, relying solely on traditional markers, like absolute neutrophil count or immunoglobulin levels, in pediatric patients with atypical or milder genotypes may overlook early or evolving immune dysfunction. Tailoring immune surveillance by age and genotype could enable earlier identification of at-risk patients and guide timely interventions, and therefore, ultimately improving outcomes across the spectrum of TBDs.
Understanding the relationship between lymphocyte deficits and infectious complications remains critical for optimizing infection risk stratification in immunocompromised populations. Importantly, in this work, decreased ALC was significantly associated with infectious complications, including opportunistic and recurrent infections, independent of age or absolute neutrophil count (see figure). This introduces a compelling idea to use ALC (<0.96 × 103/μL) as a biomarker to predict infection risk in patients with TBDs. As a routinely measured and easily accessible laboratory value, ALC could be readily implemented in this clinical context. However, applying such parameters broadly should be done with an understanding of the limitations, specifically the individual variability among patients with TBDs may limit the reliability in assessing their actual overall risk for infection. For example, this work detailed immunophenotyping, which revealed that global lymphopenia was associated with fungal infections, including Pneumocystis jirovecii. However, there is also the puzzling observation of an extremely low incidence of certain types of classic opportunistic infections, such as P jirovecii pneumonia (n = 1) despite the measurable lymphocyte deficits. This suggests that ALC, although informative, is unlikely to be sufficient to be the sole predictive factor monitored for infection risk. Rather, its interpretation must be individualized, taking into account the clinical context, disease phenotype, and other immune parameters, to guide meaningful risk stratification and prophylactic decision-making. Future prospective studies should consider incorporating assessments of lymphocyte functionality and better identifying high-risk patients, who may benefit from tailored prophylactic strategies.
This work also highlights 2 central findings that merit further investigation (see figure). First, a key observation found in this study was the association between low ALC and bone marrow failure, raising important mechanistic questions about the role of immune depletion in the hematopoietic breakdown observed in TBDs. It remains unclear whether lymphopenia is merely a surrogate for global marrow exhaustion or whether T-cell depletion directly contributes to marrow dysfunction through mechanisms such as altered immune surveillance of clonal hematopoiesis, impaired stromal support, or dysregulated cytokine signaling. T cells and their cytokine networks are increasingly recognized as key regulators of hematopoietic and stem and progenitor cell niches, and their depletion could disturb the marrow microenvironment in a way that accelerates hematopoietic decline.7 Alternatively, lymphopenia may reflect the early destruction of marrow reserve, serving as a harbinger of impending marrow collapse. Parsing causality from correlation in future prospective studies will require incorporating serial immune profiling and functional marrow assessments. Understanding whether lymphopenia drives or exacerbates marrow failure could unlock novel biomarkers and therapeutic targets in TBDs.
Second, the relationship between immune deficiency and solid tumor development in TBDs highlights a potential critical role for compromised tumor immunosurveillance in carcinogenesis. Bazzo Catto et al observed that low CD3+ and CD4+ T-cell counts were significantly associated with the development of solid tumors, most notably squamous cell carcinomas. This finding is biologically plausible, because CD4+ T cells play a central role in coordinating antitumor immune responses, including the activation of cytotoxic CD8+ T cells and the recruitment of natural killer cells and antigen-presenting cells.8 In TBDs in which telomere shortening already predisposes carriers to genomic instability and defective DNA repair, the additional loss of immune surveillance may tip the balance toward malignant transformation. Moreover, chronic inflammation and impaired clearance of oncogenic viruses, which are both consequences of immune deficiency, may further promote carcinogenesis.9 These observations raise important translational questions, such as could routine monitoring of lymphocyte subsets identify patients with a heightened cancer risk? And might early immune reconstitution, immune checkpoint blockade, or tumor surveillance protocols mitigate this risk? Addressing these questions through mechanistic studies and longitudinal clinical trials could reshape cancer prevention strategies in TBDs.
Ultimately, the work by Bazzo Catto et al reinforces the view that immunodeficiency is a key driver of morbidity and mortality in TBDs, one that is nuanced, variable, and often underestimated, particularly in adults. Tailored immune monitoring strategies that account for age, genotype, and immune phenotype may facilitate the earlier recognition of at-risk individuals and inform personalized therapeutic and prophylactic interventions. As our understanding of the immunologic landscape in TBDs continues to evolve, so too must our approach to their clinical care.
TBDs are a group of inherited conditions that are caused by germ line mutations and that effect telomere maintenance. These disorders are associated with progressive multiorgan dysfunction, including bone marrow failure, pulmonary fibrosis, and immunodeficiency. A defining feature of the immunodeficiency in adults with TBDs is lymphopenia, particularly involving low CD3+ and CD4+ T-cell counts. As shown in the left panel (blue box), lymphopenia was associated with increased infections and significantly worse overall survival. Furthermore, low lymphocyte counts correlated with higher rates of bone marrow failure and solid tumors, as illustrated in the right panel (pink box). These findings highlight the need for further investigation into how lymphopenia contributes to marrow failure and impaired tumor surveillance in TBDs.
TBDs are a group of inherited conditions that are caused by germ line mutations and that effect telomere maintenance. These disorders are associated with progressive multiorgan dysfunction, including bone marrow failure, pulmonary fibrosis, and immunodeficiency. A defining feature of the immunodeficiency in adults with TBDs is lymphopenia, particularly involving low CD3+ and CD4+ T-cell counts. As shown in the left panel (blue box), lymphopenia was associated with increased infections and significantly worse overall survival. Furthermore, low lymphocyte counts correlated with higher rates of bone marrow failure and solid tumors, as illustrated in the right panel (pink box). These findings highlight the need for further investigation into how lymphopenia contributes to marrow failure and impaired tumor surveillance in TBDs.
Conflict-of-interest disclosure: K.C.M. received clinical research funding from Elixirgen Therapeutics. J.K. declares no competing financial interests.