In this issue of Blood, Cappenberg et al1 have identified that Ras homolog family member A (RhoA) guanosine triphosphatase (GTPase)–activating protein (GAP), Myo9b, is a regulator of β2-integrin activity, which is essential for recruitment and functioning of neutrophils during inflammation.
Neutrophils are critical players in the immune response, because they are not only recruited to the inflammatory site as the first of the blood-borne leukocytes but their effector functions have a profound impact on the course of inflammation.2 These functions include pathogen phagocytosis, generation of reactive oxygen species (ROS), release of antimicrobial proteins and enzymes, and formation of neutrophil extracellular traps (NETs). All of these processes/structures facilitate the elimination of the inflammatory insult but also lead to bystander tissue and organ damage.
The recruitment of neutrophils is a multistep process following a well-recognized sequence of events: chemokine-induced activation in blood, rolling on the endothelium, firm adhesion, then crawling to the endothelial cell-cell junctions, and finally transmigration to the surrounding tissue. Eventually, chemotaxing neutrophils reach the inflammatory focus where they execute their functions.2 The above processes require integrin-ligand interactions and cytoskeleton rearrangement. Integrins are essential for both outside-in (OI) and inside-out (IO) cellular signaling. The IO signals, originating from within the neutrophil, alter integrin conformation to increase affinity for extracellular ligands, whereas OI signals transmit cues from the external environment, triggering intracellular responses such as adhesion or migration upon binding to activated integrins.3 Both processes require cytoskeleton changes facilitated by the Rho-family GTPases (Rac, Cdc42, and RhoA). Rho proteins are molecular switches cycling between inactive guanosine diphosphate–bound and active guanosine triphosphate (GTP)-bound states. Their functioning depends on RhoGEFs (promoting GTP loading to Rho) and RhoGAPs (recognizing the GTP-bound state and promoting the intrinsic GTPase activity). Active Rhos adopt a conformation that enables coupling to effector proteins that propagate signal transduction events.4 Of the 3 Rho proteins, RhoA enhances actomyosin-II contractility and cell retraction, and is known to be negatively regulated by the myosin Myo9b, a RhoGAP.
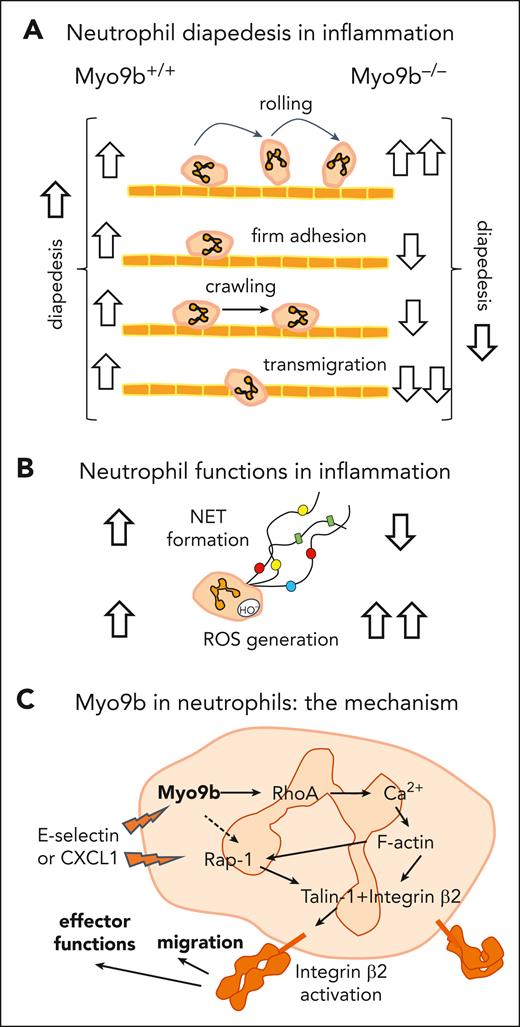
Considering both the benefits and drawbacks of neutrophil recruitment and function in inflammatory disorders, Cappenberg et al explored the impact of Myo9b on β2 integrins, which are exclusively expressed on leukocytes and are essential for neutrophil diapedesis.3 Using Myo9b–/– knockout (KO) mice, the group demonstrated, in 2 models of inflammation (sterile ischemia-reperfusion injury and bacterial sepsis), that the absence of Myo9b compromises neutrophil recruitment to the inflammatory site without affecting other leukocytes. In this KO model phenotype, the bystander damage was attenuated. The neutrophil recruitment cascade was further investigated ex vivo and through intravital imaging of the cremaster muscle vasculature in KO mice and their bone marrow chimeras with wild-type animals. The studies revealed a mixed impact of the RhoGAP on each step of neutrophil diapedesis, ultimately resulting in an overall impairment of the process (see figure panel A). Furthermore, Cappenberg et al demonstrated that Myo9b deficiency leads to RhoA overactivation, consistent with previous observations in macrophages.5 This underscores the importance of Myo9b in orchestrating an appropriate inflammatory response and preventing RhoA hyperactivity in different leukocyte populations.
Major findings of Cappenberg et al on the role of Myo9b in neutrophils. Major findings of Cappenberg et al on the role of Myo9b in neutrophil migration (A) and effector functions (B) as well as the mechanism underlying its regulatory role involving integrin β2 (C). Studies were carried out on Myo9b KO–/– and wild-type+/+ mice. ↑, increased; ↓, decreased; dashed line, partial impact. CXCL1, chemokine (C-X-C motif) ligand 1; Rap-1, Ras-related protein 1.
Major findings of Cappenberg et al on the role of Myo9b in neutrophils. Major findings of Cappenberg et al on the role of Myo9b in neutrophil migration (A) and effector functions (B) as well as the mechanism underlying its regulatory role involving integrin β2 (C). Studies were carried out on Myo9b KO–/– and wild-type+/+ mice. ↑, increased; ↓, decreased; dashed line, partial impact. CXCL1, chemokine (C-X-C motif) ligand 1; Rap-1, Ras-related protein 1.
Cappenberg et al also investigated selected neutrophil functions, specifically ROS and NET formation, in Myo9b–/– mice, finding that these processes were increased and decreased, respectively (see figure panel B). Cytoskeletal rearrangement is indeed required for NET formation, but NET triggering often depends on ROS, and so do the cytoskeletal dynamics accompanying trap formation.6 This ambiguity, with Myo9b exerting different effects on ROS and NETs, warrants further investigation. The involvement of Rho GTPases in NET formation has scarcely been studied, and, specifically, not in the context of Myo9, with the studies reporting divergent findings.7,8 Given the ongoing need to limit excessive NET formation during inflammation, because of its long-term detrimental effects,9 further studies using biologically relevant NET inducers should be spurred by the current findings.
An important aspect of Cappenberg et al study was the demonstration that Myo9b regulates neutrophil β2-integrin affinity and valency induced by either a chemokine (chemokine [C-X-C motif] ligand 1) or selectins. The study also showed that Myo9b controls chemokine-induced calcium flux into neutrophils, thereby influencing actin polymerization (F-actin levels), which is necessary for the recruitment of talin-1 to β2 integrins. Talin-1 binds to the integrin β cytoplasmic domain, leading to its activation.10 Cappenberg et al demonstrated that chemokine-/selectin-induced activation of Ras-related protein 1 GTPase, which governs this process, is at least partially Myo9b dependent, thereby outlining the mechanisms by which Myo9 affects RhoA in neutrophils (see figure panel C).
Given that both IO and OI integrin-dependent processes in neutrophils are regulated by Myo9b and that these cells are pivotal to the development and execution of the inflammatory response, including both its beneficial (resolution) and harmful (collateral damage) aspects, Myo9b emerges as an important regulator of inflammation. As such, it could be considered a possible therapeutic target in inflammatory disorders.
Conflict-of-interest disclosure: E.K. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal