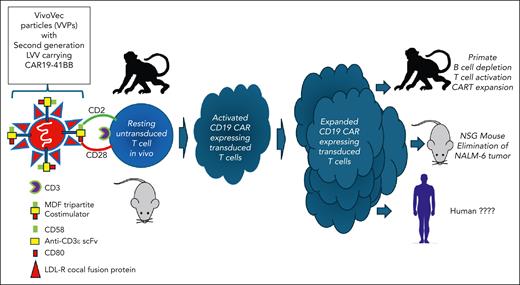
In this issue of Blood, Nicolai et al describe the use of novel serum resistant pseudotyped viral particles for in vivo chimeric antigen receptor T-cell (CART) therapy for targeting CD19 in mice and primates.1 Traditional ex vivo gene therapy for the generation of CARTs requires many lengthy and costly steps before an autologous product is ready for infusion. This process can both fail and be inefficient. The authors develop several novel reagents and platforms for in vivo gene therapy eliminating the need for apheresis, shipping, lengthy central ex vivo processing and T-cell activation, multiple cryopreservation steps, and most importantly, the need for lymphodepletion. This platform and others using adeno-associated virus (AAV), pseudotyped lentiviruses, and lipid nanoparticles for the in vivo transduction of both T cells and hematopoietic stem cells could change everything. Proving it will be the challenge.
So, is the sun rising on a new world of in vivo CART therapy or is this the beginning of the end for traditional ex vivo CART platforms for the treatment of B-cell malignancies? Perhaps one of the best examples of the progress made in this field is the work of Nicolai and colleagues featuring a novel third-generation lentiviral vector (LVV), VivoVec, engineered to express both T-cell targeting protein (CD3ε single-chain variable fragment [scFv]) and T-cell costimulatory ligands (CD80 and CD58) for optimal costimulation, activation, and in vivo transduction via engagement of CD28/CTLA-4 and CD2, respectively. Human embryonic kidney (HEK)-293T cells are transduced with 5 plasmids (gag, pol, rev, cocal, MDF, and LV containing the CD19 CAR payload). Herein lies the secret sauce. First, the particles are pseudotyped with the low density lipoprotein receptor (LDL-R)–tropic cocal fusion glycoprotein as a substitute for VSV-G, which is more resistant to inactivation by human serum allowing for in vivo administration.2 Second, in vivo activation of resting T cells via CD3 scFv and costimulation with CD80/CD58 or by tripartite fusion of CD3ε scFv, CD80, and CD58 (“MDF”) is critical for optimal biologic effect. Costimulation, especially with MDF, results the most effective in vivo activation and transduction of resting T cells, obviating the need for strong ex vivo activation with CD3×CD28 beads (see figure).
Proposed schema for in vivo CART therapy using LDL-R cocal fusion protein pseudotyped virus expressing novel T-cell targeting elements (anti-CD3ε scFv) and costimulatory molecules (CD58 and CD80).
Proposed schema for in vivo CART therapy using LDL-R cocal fusion protein pseudotyped virus expressing novel T-cell targeting elements (anti-CD3ε scFv) and costimulatory molecules (CD58 and CD80).
The authors provide detailed and convincing evidence that this is an effective platform in vivo using NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse models. They show that increasing doses of viral particles injected intraperitoneally (IP) in NSG mice that were previously engrafted with human peripheral blood mononuclear cells IP result in robust activation and transduction of human T cells expressing CD19 CAR in vivo, which is optimal when costimulation with the MDF expressing particles are used resulting in a dose-dependent control of CD19+ NALM-6 tumor growth in vivo.
The authors then performed a parallel set of experiments in nonhuman primates (NHPs) where they had to switch the scFv from human CD19 to human/primate CD20 since there is no known anti-human CD19 scFv that cross-reacts with the NHP CD19. In these pivotal experiments they were able show a dramatic, dose-dependent, and durable (>45 days) elimination of B cells in 3 of 4 NHPs and with 25% to 65% transduction efficiency in vivo by day 11 and with >50% of the cells in the blood being CART and with absolute CART per microliter of blood reaching >10 000/μL. Although cytokine release syndrome (CRS) seemed quite minimal, it is not clear how CRS in NHPs can be extrapolated to humans. VivoVec particles were injected directly into the lymph nodes of the NHP and not the blood. Was this because intravenous injection did not work as effectively? Of note in the NHPs with the longest B-cell aplasia, biodistribution studies demonstrated transduced cells only in the injected and regional lymph nodes and nowhere else, which is reassuring. Of interest is that this technology is heading for the clinic in 2024 using UB-VV111, a lentivirus-based in vivo generated anti-CD19 CART for the treatment of patients with chronic lymphocytic leukemia and large B-cell lymphoma, which uses a built-in rapamycin-activated interleukin-2 (IL-2)/IL-15 signaling to promote expansion and persistence while preventing host immune-based elimination of CART.3
What does it mean if these studies can be recapitulated in humans? Well, a lot! The complexity of generating and manufacturing CART ex vivo as well as the time involved resulted in some patients dying while waiting for treatment; the need for lymphodepletion and its associated toxicities and the costs are enormous. If successful and if proven to be safe and at least as effective, then this could be a game changer, already several in vivo gene therapy products have already been approved by the Food and Drug Administration including Luxturna for biallelic RPE65 mutated retinal dystrophy4 and Elevidys for Duchenne muscular dystrophy (NCT05096221).
It should be noted that there is increasing competition in this narrow space. In the last several years at least 5 start-up companies are pursuing in vivo gene therapy in the cancer field using multiple vectors (AAV, lipid nanoparticles [LNPs], pseudotyped lentiviral particles) and platforms including Umoja Biopharma, Capstan Therapeutics, Interius BioTherapeutics, Ensoma, and Vector BioPharma. This approach may have significant advantages for stem cell gene therapy as well. Investigators from the University of Pennsylvania successfully targeted circulating and bone marrow CD117+ stem cells with LNPs resulting in efficient CRISPR/Cas-based correction of hematopoietic stem cells bearing the sickle cell mutation.5 The ability to target hematopoietic stem cell or T cells in vivo with either RNA containing LNPs or pseudotyped LVVs provides not only a nongenotoxic conditioning regimen for hematopoietic stem cell transplantation, gene transfer, and gene editing but no conditioning at all, which is a major step forward.6,7
So, is this the dawn of a new age or the end of an era? This commentary may seem a bit Pollyannaish focusing on the glitter and potential of in vivo gene therapy, but there are plenty of potential risks. Will these therapies be actually safer when compared with ex vivo gene therapy? Only time will tell. Will they work as well or better? Well, big studies may be the only way to answer this question, but who will have the juice or the funding to get these comparator studies done? Will they be cheaper to administer to patients? Well, if the history of drug development has taught us anything, then sadly, I must say that this is unlikely.
Conflict-of-interest disclosure: J.F.D. declares an equity ownership and research funding from WUGEN.