In this issue of Blood, Schyrr et al report the development of a mouse model of extramedullary hematopoiesis (EMH) in the adrenal glands, which was useful for revealing the mechanism of hematopoiesis.1
Clarification of the mechanisms involved in hematopoiesis are important to understand the generation and differentiation of hematopoietic stem cells (HSCs) into red blood cells, white blood cells, platelets, and neoplasms. In addition, understanding the diverse potential niches involved in hematopoiesis are important for developing new therapies to combat leukemogenesis, drug resistance, and the recurrence of leukemia.2 Hematopoiesis resides primarily in bone marrow in adult humans. EMH is mostly commonly observed in the spleen and liver. In adult humans, splenic EMH is observed under the condition of the suppression of the bone marrow space by severe cancer metastasis to the bones, for example. In rare cases, EMH can be observed in lymph nodes, kidneys, thoracic spaces, cranial duras, and other organs.3
CXCL12 is well known as a key regulator of the bone marrow niche, impacting stromal cells, such as CXCL12-abundant reticular cells, and HSCs.4 Similarly, CXCL12 plays a critical role in EMH in the spleen,5 with upregulation of CXCL12 in sinusoidal endothelial cells observed in the EMH in the spleen.6
EMH can be exploited to explore the genesis and mechanism of hematopoiesis and niches as researchers can create 2 experimental conditions in niches: one with hematopoiesis and one without. Researchers can create conditions for identifying and validating the effects of specific molecules with and without EMH. One major barrier to this approach is that mice, even adults, have physiological EMH in the spleen. This is a major difference from humans. Thus, an inducible EMH model in mice would be better to assess the niche and impacts of external factors on the niche and hematopoiesis.
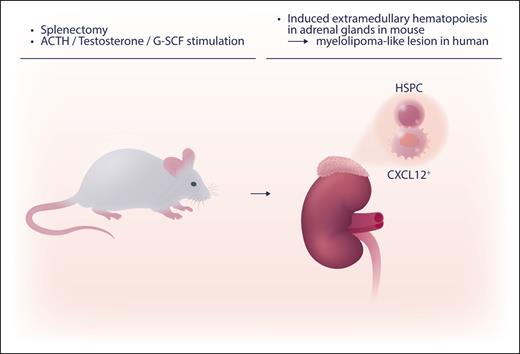
The new mouse model of EMH in the adrenal glands by Schyrr et al first requires splenectomy followed by hormonal stimulation. A cocktail of G-CSF (granulocyte colony-stimulating factor), testosterone, and ACTH (adrenocorticotropic hormone) is injected into mice (see figure). HSCs and progenitor cells are recruited to the EMH foci by the CXCL12/CXCR4 axis. More research is needed to further elucidate the mechanisms involved in the induction of adrenal EMH. The CXCL12/CXCR4 axis has been well studied. The upstream regulator of the expression of CXCL12 was recently reported in an in vitro microenvironmental model.7 This in vivo EMH model will be useful when used with other murine analytic systems, such as knockout mouse backgrounds, to explore the genesis of EMH in vivo.
Inducible EMH in adrenal glands was induced by splenectomy, followed 10 days later by daily injection of hormonals (the cocktail of G-CSF, testosterone, and ACTH) for 20 to 21 days. HSPC, hematopoietic stem and progenitor cells. Professional illustration by Somersault18:24.
Inducible EMH in adrenal glands was induced by splenectomy, followed 10 days later by daily injection of hormonals (the cocktail of G-CSF, testosterone, and ACTH) for 20 to 21 days. HSPC, hematopoietic stem and progenitor cells. Professional illustration by Somersault18:24.
This inducible EMH is morphologically suggestive of human adrenal myelolipoma. Myelolipoma is not a common disease, but histologically hematopoietic cells and adipocytes like found in the bone marrow are observed in the adrenal gland. The etiology is not entirely clear, but human myelolipoma have been reported where there was hormonal augmentation (high ACTH).8 Schyrr et al found the structural resemblance of human myelolipoma to the mouse adrenal EMH model, but in this murine model, adipose tissue, did not proliferate. Further work is needed to explore CXCL12+ stromal cells in human myelolipoma. The exploration of the mechanism of hematopoiesis using this new EMH model may also help our understanding of the recovery and repopulation of blood cells and, thereby, impact treatment for hematopoietic neoplasm.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal