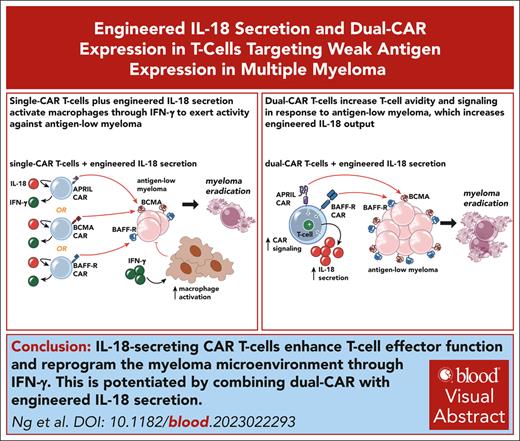
Key Points
IL-18-secreting CAR T cells promote clearance of antigen-low myeloma with reprogramming of cells in the myeloma microenvironment.
Multiantigen targeting enhances T-cell-target avidity, increasing engineered IL-18 output and potentiating antimyeloma activity.
Visual Abstract
Multiple myeloma is a plasma cell malignancy that is currently incurable with conventional therapies. Following the success of CD19-targeted chimeric antigen receptor (CAR) T cells in leukemia and lymphoma, CAR T cells targeting B-cell maturation antigen (BCMA) more recently demonstrated impressive activity in relapsed and refractory myeloma patients. However, BCMA-directed therapy can fail due to weak expression of BCMA on myeloma cells, suggesting that novel approaches to better address this antigen-low disease may improve patient outcomes. We hypothesized that engineered secretion of the proinflammatory cytokine interleukin-18 (IL-18) and multiantigen targeting could improve CAR T-cell activity against BCMA-low myeloma. In a syngeneic murine model of myeloma, CAR T cells targeting the myeloma-associated antigens BCMA and B-cell activating factor receptor (BAFF-R) failed to eliminate myeloma when these antigens were weakly expressed, whereas IL-18–secreting CAR T cells targeting these antigens promoted myeloma clearance. IL-18-secreting CAR T cells developed an effector-like T-cell phenotype, promoted interferon-gamma production, reprogrammed the myeloma bone marrow microenvironment through type-I/II interferon signaling, and activated macrophages to mediate antimyeloma activity. Simultaneous targeting of weakly-expressed BCMA and BAFF-R with dual-CAR T cells enhanced T-cell:target-cell avidity, increased overall CAR signal strength, and stimulated antimyeloma activity. Dual-antigen targeting augmented CAR T-cell secretion of engineered IL-18 and facilitated elimination of larger myeloma burdens in vivo. Our results demonstrate that combination of engineered IL-18 secretion and multiantigen targeting can eliminate myeloma with weak antigen expression through distinct mechanisms.
Introduction
Multiple myeloma is a disease of transformed plasma cells that is located primarily in the bone marrow (BM). This malignancy is characterized by excessive immunoglobulin production, osteolytic bone lesions, and increased susceptibility to infection.1 Although recent decades have yielded several life-extending therapies, the disease remains incurable with a 5-year survival rate just below 60%.2 Current treatments include chemotherapy, hematopoietic stem-cell transplantation, proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, and most recently bispecific T-cell engagers and chimeric antigen receptor (CAR) T cells.3-6 Following the success of CD19-CAR T cells in B-cell malignancies, the US Food and Drug Administration recently approved 2 B-cell maturation antigen (BCMA)–directed CAR T-cell products in relapsed and refractory multiple myeloma.7-10 However, emerging data indicate that BCMA can be weakly expressed at baseline, downmodulated, genetically deleted over the disease course, or mutated to subvert BCMA single-chain fragment variable (of antibody) (scFv) binding, rendering the myeloma insensitive to BCMA-directed therapy.11-15
Strategies to overcome variable antigen expression levels in malignant cells include engineered proinflammatory cytokine secretion to activate endogenous immunity,16-18 dual-targeting of 2 tumor-associated antigens with CARs19 or chimeric costimulatory receptors to augment CAR signaling,20,21 enhancing CAR signaling via CD28 costimulation,22,23 and leveraging the endogenous T-cell receptor to enhance CAR sensitivity.24 Alternative myeloma-associated antigens alongside BCMA include transmembrane activator and CAML activator (TACI), signaling lymphocyte activation molecule family member 7 (SLAMF7), G-protein coupled receptor family C group 5 member D (GPRC5D), CD229, CD138, and CD38.4,25-30 Myeloma can also express B-cell activating factor receptor (BAFF-R) and CD19.31-33 Multiantigen targeting is achieved by coexpressing several CARs, bispecific tandem-CARs,34,35 or ligand-based CARs as receptor binding domains.36,37 Engineering T cells to recognize several antigens can enable elimination of tumor cells by targeting an alternatively expressed antigen38 or simultaneous targeting of multiple weakly-expressed antigens,19 summing multiple CARs signals to sufficiently activate T cells.
Engineered secretion of the proinflammatory cytokine interleukin-18 (IL-18) can enhance activation of CAR T cells or bystander immune cells.17,18,39 Further, CD19-CAR T cells secreting IL-18 have demonstrated early clinical activity (NCT04684563).40 Because weak antigen expression on malignant cells can promote insufficient CAR T-cell activation, we hypothesized that engineered IL-18 secretion and multiantigen targeting could be each employed to improve CAR T-cell activity antigen-low myeloma by enhancing T-cell activation and stimulating endogenous immune cells.
We utilized syngeneic CAR T cells to target 3 tumor necrosis factor-superfamily receptors that are variably expressed in myeloma and important for growth31: BCMA and TACI, (both bind a proliferation inducing ligand [APRIL]-ligand), and BAFF-R. Although most preclinical myeloma–directed CAR T-cell studies have employed xenograft models, we used an immunocompetent mouse model of multiple myeloma to characterize the microenvironment following CAR T-cell treatment. Although CAR T cells targeting BCMA or BAFF-R failed to eliminate antigen-low myeloma in vivo, engineered IL-18 secretion enhanced T-cell activity and enabled myeloma clearance. APRIL-CAR T cells secreting IL-18 promoted type-I/II interferon signaling in T cells and myeloid cells, activating macrophages to facilitate eradication of antigen-low myeloma. Finally, combination of engineered IL-18 secretion with BAFF-R/APRIL dual-CAR coexpression enhanced T-cell activity against BCMAlowBAFF-Rlow myeloma. Dual-CAR expression enhanced T-cell cytotoxicity and increased engineered IL-18 secretion upon recognition of antigen-low targets. IL-18–secreting dual-CAR T cells represent a multifaceted strategy to eliminate antigen-low disease by stimulating CAR T-cell cytotoxicity and endogenous immune activation.
Methods
Full description of methods can be found in supplemental Information, available on the Blood website.
DNA constructs were generated using standard molecular biology techniques, then cloned into viral vectors and transfected into Phoenix-E–based virus packaging lines. Viral supernatant was filtered and concentrated with polyethylene glycol.
For T-cell transduction, BALB/c splenic T cells were isolated through B-cell depletion with Miltenyi murine-CD19 microbeads, then stimulated with anti-CD3 and anti-CD28. On day 1, concentrated viral supernatant was spun onto an anti-CD3/28 and retronectin coated plate before adding stimulated T cells. On day 2, CAR/vector expression was measured with flow cytometry and cells were stimulated for 1 additional day or retransduced. Transduced T cells were magnetically sorted on day 3 with Miltenyi LS columns and microbeads specific for the appropriate expression tags. Column-bound cells were eluted into Roswell Park Memorial Institute complete media with IL-2. For in vitro assessment, CAR T cells were cocultured with MOPC315.BM at varied effector-to-target (E:T) ratios on day 4. Data were collected from 24 hours to 120 hours later with a luciferase–based viability assay or the Incucyte SX5.
For animal experiments, BALB/c mice were lymphodepleted with 450 cGy of sublethal irradiation and injected intravenously with MOPC315.BM. Three days later, CAR T cells were administered retro-orbitally (day 5 after transduction). For tumor bioluminescent imaging (BLI), mice were intraperitoneally injected with D-luciferin and imaged 10 minutes later. For T-cell BLI, mice were injected retro-orbitally with water-soluble coelenterazine and imaged immediately. For ex vivo analysis, BM of CAR T-cell–treated mice was harvested on day 7 after tumor (day 4 after T cells). Peripheral blood collection was performed retro-orbitally. BM aspirates were performed on live mice.
Results
Development of syngeneic models of multiple myeloma with varied antigen expression
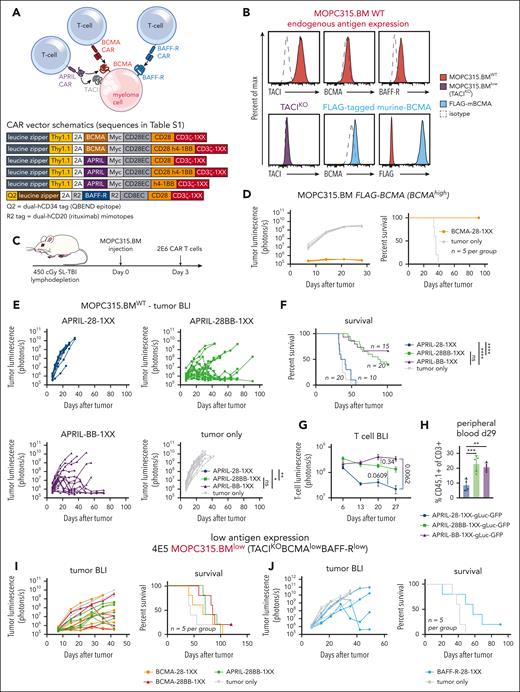
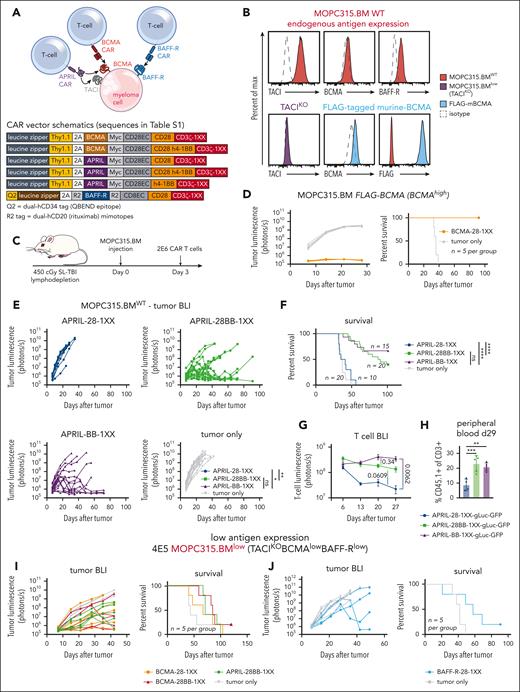
To study myeloma–directed CAR T cells in a syngeneic and immunocompetent model, we utilized MOPC315.BM,41 a BALB/c plasmacytoma cell line that recapitulates clinical myeloma features in vivo-BM infiltration (supplemental Figure 1) and clinical sequelae such as osteolytic lesions and paraplegia. This cell line can be genetically modified in vitro and maintain tumorigenicity in mice. To evaluate CAR T-cell activity against weak expression of several of myeloma-associated antigens, we targeted murine BCMA/TACI with an APRIL-ligand–based CAR, and BCMA or BAFF-R with scFv-based CARs (Figure 1A). Human versions of these CAR T cells have demonstrated activity in xenograft models.36,42-45
Myeloma–directed CAR T cells are impaired by low antigen expression in vivo. (A) Schematic of the APRIL-CAR targeting both BCMA/TACI and scFv–based BCMA and BAFF-R CARs targeting BCMA and BAFF-R, respectively, on a myeloma cell. All vector maps for CARs used in this figure are depicted below. (B) Endogenous expression of TACI, BCMA, and BAFF-R on MOPC315.BM (top, red). Validation of TACI KO with CRISPR-Cas9-gRNA electrporation (purple), and overexpression of a FLAG–tagged murine BCMA that was expressed with a transposase (blue). (C) Schematic of syngeneic MOPC315.BM in vivo model. Mice were lymphodepleted with 450 cGy sublethal total body irradiation (SL-TBI) on day 0 and then injected tail IV with MOPC315.BM. CAR T cells were injected 3 days later. (D) Tumor BLI and survival of mice bearing 4E5 MOPC315.BM FLAG-mBCMA (overexpressed) and treated with BCMA-28-1XX T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BM FLAG-mBCMA. 2E6 CAR T cells were injected 3 days later. BCMA-CAR T cells were cultured with 1 μM dasatinib for 2 days prior to injection into mice. (E-F) Tumor BLI and survival of mice bearing MOPC315.BMWT (TACIhighBCMAlowBAFF-Rlow) and treated with APRIL-CAR T cells with varied costimulatory domains. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. BLI statistics performed with Vardi area under the curve (AUC) analysis and survival statistics with Mantel-Cox log-rank test. Data are combined from at least 4 independent experiments. (G) T-cell BLI of mice treated with CD45.1+ APRIL-CAR T cells labeled with gLuc-GFP. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. T cells were imaged weekly starting on day 6. Statistics were performed with the 1-way analysis of variance (ANOVA) test on day 27. Data are combined from at least 2 independent experiments. (H) CD45.1+ T-cell abundance measured in the peripheral blood of mice from panel G on day 29, as measured by flow cytometry. Statistics were performed with the 1-way ANOVA test. Data are representative of at least 2 independent experiments. (I-J) Tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with APRIL, BCMA, or BAFF-R-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Myeloma–directed CAR T cells are impaired by low antigen expression in vivo. (A) Schematic of the APRIL-CAR targeting both BCMA/TACI and scFv–based BCMA and BAFF-R CARs targeting BCMA and BAFF-R, respectively, on a myeloma cell. All vector maps for CARs used in this figure are depicted below. (B) Endogenous expression of TACI, BCMA, and BAFF-R on MOPC315.BM (top, red). Validation of TACI KO with CRISPR-Cas9-gRNA electrporation (purple), and overexpression of a FLAG–tagged murine BCMA that was expressed with a transposase (blue). (C) Schematic of syngeneic MOPC315.BM in vivo model. Mice were lymphodepleted with 450 cGy sublethal total body irradiation (SL-TBI) on day 0 and then injected tail IV with MOPC315.BM. CAR T cells were injected 3 days later. (D) Tumor BLI and survival of mice bearing 4E5 MOPC315.BM FLAG-mBCMA (overexpressed) and treated with BCMA-28-1XX T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BM FLAG-mBCMA. 2E6 CAR T cells were injected 3 days later. BCMA-CAR T cells were cultured with 1 μM dasatinib for 2 days prior to injection into mice. (E-F) Tumor BLI and survival of mice bearing MOPC315.BMWT (TACIhighBCMAlowBAFF-Rlow) and treated with APRIL-CAR T cells with varied costimulatory domains. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. BLI statistics performed with Vardi area under the curve (AUC) analysis and survival statistics with Mantel-Cox log-rank test. Data are combined from at least 4 independent experiments. (G) T-cell BLI of mice treated with CD45.1+ APRIL-CAR T cells labeled with gLuc-GFP. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. T cells were imaged weekly starting on day 6. Statistics were performed with the 1-way analysis of variance (ANOVA) test on day 27. Data are combined from at least 2 independent experiments. (H) CD45.1+ T-cell abundance measured in the peripheral blood of mice from panel G on day 29, as measured by flow cytometry. Statistics were performed with the 1-way ANOVA test. Data are representative of at least 2 independent experiments. (I-J) Tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with APRIL, BCMA, or BAFF-R-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Flow cytometric assessment of endogenous MOPC315.BM (MOPC315.BMWT) antigen expression revealed substantial expression of TACI but weak BCMA and BAFF-R (Figure 1B, red). To further motivate our study of BCMA, TACI, and BAFF-R, we assessed expression of these antigens from a publicly available single-cell RNA sequencing (scRNAseq) data set of 20 relapsed and refractory myeloma patients.46 BCMA, TACI, and BAFF-R expression was present in myeloma samples; however, we observed both weak expression of these antigens in different patients and heterogeneous expression within samples (supplemental Figure 2A), as has been previously reported.11,12,31,32 This variable antigen expression in patients parallels weak BCMA and BAFF-R expression in MOPC315.BM.
To establish an antigen-low myeloma model, we electroporated recombinant CRISPR-Cas9+TACI-guide RNA ribonucleoprotein-complexes to knockout TACI (Figure 1B, purple), resulting in MOPC315.BM endogenously expressing only weak BCMA and BAFF-R. These cells, denoted MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow), restricted the APRIL-CAR to recognize only BCMA. To further compare CAR T-cell responses against myeloma targets with weak vs strong BCMA expression, we overexpressed BCMA in MOPC315.BMlow using a FLAG–tagged (amino acid sequence DYKDDDDK) murine-BCMA construct (MOPC315.BM-TACIKOFLAG-mBCMA, Figure 1B, blue).
Low target antigen density impairs the activity of myeloma–directed CAR T cells in vivo
We first constructed CD28-costimulated BCMA and APRIL-CARs, as CD28 costimulation has been reported to perform better against weak antigen expression.22 APRIL-28ζ and scFv–based BCMA-28ζ T cells bound soluble murine BCMA similarly (supplemental Figure 2B) and eliminated BCMA-overexpressing MOPC315.BM-TACIKOFLAG-mBCMA equivalently in vitro (supplemental Figure 2C). APRIL-CAR T cells also eliminated TACIhigh-MOPC315.BMWT. Conversely, these CAR T cells both required >10× higher E:T ratio to lyse >50% MOPC315.BMlow, confirming that CAR T-cell killing of BCMA+ myeloma is antigen density dependent.
To further evaluate CAR designs before testing in vivo, we designed APRIL and BCMA CARs with inactivating mutations in the second/third immunoreceptor tyrosine–based activation motifs (ITAMs) of CD3ζ (denoted 1XX), which are reported to enhance CAR T-cell activity in vivo.47 We observed that scFv–based BCMA-28ζ T cells failed to control MOPC315.BM at a low E:T ratio over several days, while APRIL-28ζ T cells killed this line effectively (supplemental Figure 3A). In contrast, BCMA-28-1XX T cells enhanced MOPC315.BM lysis in this setting. BCMA-28ζ T cells cultured without antigen tonically upregulated inhibitory receptors, suggesting T-cell dysfunction caused by tonic CAR signaling.47,48 This was attenuated with 1XX ITAM mutations and greatly ameliorated by adding the kinase inhibitor dasatinib to culture, which blocks tonic CAR signaling (supplemental Figure 3B).49 Dasatinib–cultured BCMA-28-1XX T cells achieved complete responses against MOPC315.BM-FLAG-mBCMA in mice (Figure 1D). Therefore, we used both the CD3ζ-1XX mutant and dasatinib culture for all scFv–based CAR T cells, to attenuate tonic-signaling–induced dysfunction.
In contrast to scFv–based BCMA CARs, APRIL ligand–based CARs promoted less tonic upregulation of inhibitory receptors (supplemental Figure 3C). However, APRIL-CAR T cells failed to control TACIhigh-MOPC315.BMWT in vivo, with neither ITAM attenuation nor dasatinib preculture improving activity (supplemental Figure 3D-F). 4-1BB costimulation can promote CAR T-cell persistence, attenuate exhaustion, and promote favorable metabolism.48,50-53 Therefore, we modified APRIL-CARs with either third-generation CD28/4-1BB costimulation (28BB) or second-generation 4-1BB costimulation, along with 1XX ITAMs. In vitro analysis revealed that APRIL-28BB-1XX T cells lysed targets slightly better than APRIL-28BBζ T cells (supplemental Figure 4A), whereas the 4-1BB T-cell configurations were less lytic. 4-1BB costimulation dominantly enhanced APRIL-CAR T-cell activity against MOPC315.BMWT in vivo, as the CD28-4-1BB or 4-1BB configurations increased survival of mice (Figure 1E-F). T-cell BLI demonstrated that CD28-4-1BB or 4-1BB costimulation promoted superior persistence of APRIL-CAR T cells compared with CD28 costimulation (Figure 1G). This finding was corroborated by increased CAR T-cell frequency in the peripheral blood of 4-1BB-costimulated CAR T-cell recipients (Figure 1H; supplemental Figure 4B-C).
Despite the potent antimyeloma activity observed against antigen-high myeloma, BCMA-28-1XX and third-generation BCMA-28BB-1XX or APRIL-28BB-1XX T cells all failed to promote long-term survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) (Figure 1I). Similarly, CD28-costimulated BAFF-R-28-1XX T cells most effectively lysed BAFF-Rlow-MOPC315.BM in vitro but failed to generate long-term survival in vivo (supplemental Figure 4D, 1J). These findings mirror clinical observations that insufficient antigen expression limits CAR T-cell activity and highlights the need for additional interventions to effectively address antigen-low myeloma.
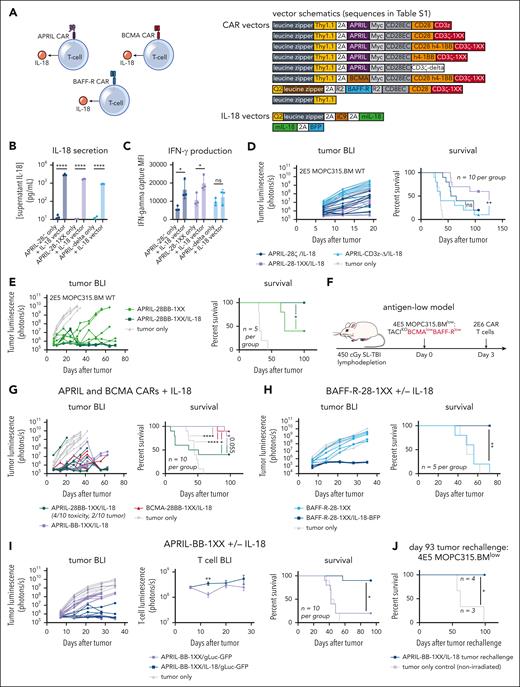
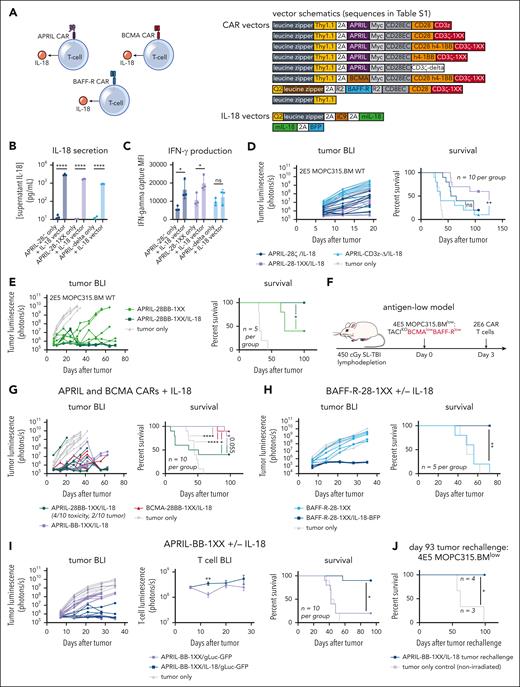
Engineered IL-18 secretion promotes clearance of antigen-high and antigen-low myeloma in vivo
Several studies have demonstrated that engineered secretion of the proinflammatory cytokine IL-18 enhances CD28–costimulated CAR T-cell activity and activates bystander immune cells.17,18,39 Therefore, we coexpressed IL-18 in T cells alongside myeloma-directed CARs to potentially enhance activity against antigen-low myeloma (Figure 2A). We transduced IL-18 into T cells on a separate vector coexpressing the safety-switch iCaspase 9, and used our Zip-sorting technology to magnetically purify double-transduced cells (supplemental Figure 5A-B).54 We confirmed IL-18 secretion and stimulation of interferon-gamma (IFN-γ) production in CAR T cells (Figure 2B-C). IL-18 secretion enhanced the activity of CD28–costimulated APRIL-CAR T cells against TACIhigh-MOPC315.BMWT in vivo, with APRIL-28-1XX/IL-18 producing more long-term survivors than APRIL-28ζ/IL-18 (Figure 2D). Nonsignaling APRIL-Δ/IL-18 T cells did not produce substantial long-term survival, indicating that IL-18–secreting T cells require CAR signaling to eliminate myeloma. To further assess the specificity of IL-18–secreting T cells, we constructed a CD19-CAR to determine if IL-18 secretion promotes antigen-independent activity against CD19-negative MOPC315.BMWT. We first validated CD19-28-1XX T-cell activity against MOPC315.BM modified to overexpress murine-CD19 (MOPC315.BM-CD19+, supplemental Figure 5C-D). Next, we challenged CD19-28-1XX/IL-18 T cells in vivo with a mixed population of CD19-negative MOPC315.BMWT and CD19-positive MOPC315.BM-CD19+ to stimulate IL-18–secreting CAR T cells in the presence of the CD19-negative population. CD19-28-1XX/IL-18 T cells extended survival in this setting but did not cure mice (supplemental Figure 5E), indicating IL-18 secretion alone is insufficient to clear CD19-negative MOPC315.BM. This suggests that ongoing engagement of CAR and surface antigen is required for antimyeloma activity with IL-18–secreting CAR T cells.
Engineered IL-18 secretion by CAR T cells improves therapeutic activity against antigen-high and antigen-low myeloma. (A) Schematic of APRIL, BCMA, or BAFF-R CAR T cells engineered to secrete IL-18. Vector maps for CAR constructs used in this figure are depicted to the right. (B) Engineered IL-18 secretion levels by APRIL-CAR T cells ± engineered IL-18 secretion. Approximately 100 000 CAR T cells were cultured for 24 hours in IL-2 and the supernatant was collected for ELISA. Concentration was determined based on standard curve measurements. Statistical analysis performed with the Student t test. (C) IFN-γ secretion by APRIL-CAR T cells ± engineered IL-18 secretion. Supernatant from setup in panel B was analyzed using an IFN-γ flow cytometric bead array kit. Statistical analysis performed with the Student t test. (D) Tumor BLI and survival of mice bearing MOPC315.BMWT and treated with CD28-costimulated, IL-18-secreting APRIL-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. Data are combined from at least 2 independent experiments. (E) Tumor BLI and survival of mice bearing MOPC315.BMWT and treated with CD28-4-1BB-costimulated IL-18-secreting APRIL-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. (F) Schematic of syngeneic antigen-low MOPC315.BMlow in vivo model (used in panels G-I). Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. (G) Tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with IL-18-secreting APRIL or BCMA-CAR T cells. Tumor BLI was performed weekly starting on day 7. Data are combined from at least 2 independent experiments. (H) Tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with BAFF-R-28-1XX CAR T cells ± IL-18. Tumor BLI was performed weekly starting on day 7. (I) Tumor BLI, T-cell BLI, and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with APRIL-BB-1XX/IL-18 T cells compared with control APRIL-BB-1XX T cells without engineered IL-18 expression. T-cell BLI was performed weekly starting on day 6. Tumor BLI was performed weekly starting on day 7. Data are combined from at least 2 independent experiments. (J) Survival of APRIL-BB-1XX/IL-18 treated complete responder mice that were rechallenged with 4E5 MOPC315.BM TACIKO(BCMAlowBAFF-Rlow) 93 days after original experiment start date. Mice were not irradiated before re-challenge. Statistical analysis of all survival curves was performed with the Mantel-Cox log-rank test. ELISA, enzyme-linked immunosorbent assay. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Engineered IL-18 secretion by CAR T cells improves therapeutic activity against antigen-high and antigen-low myeloma. (A) Schematic of APRIL, BCMA, or BAFF-R CAR T cells engineered to secrete IL-18. Vector maps for CAR constructs used in this figure are depicted to the right. (B) Engineered IL-18 secretion levels by APRIL-CAR T cells ± engineered IL-18 secretion. Approximately 100 000 CAR T cells were cultured for 24 hours in IL-2 and the supernatant was collected for ELISA. Concentration was determined based on standard curve measurements. Statistical analysis performed with the Student t test. (C) IFN-γ secretion by APRIL-CAR T cells ± engineered IL-18 secretion. Supernatant from setup in panel B was analyzed using an IFN-γ flow cytometric bead array kit. Statistical analysis performed with the Student t test. (D) Tumor BLI and survival of mice bearing MOPC315.BMWT and treated with CD28-costimulated, IL-18-secreting APRIL-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. Data are combined from at least 2 independent experiments. (E) Tumor BLI and survival of mice bearing MOPC315.BMWT and treated with CD28-4-1BB-costimulated IL-18-secreting APRIL-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E5 MOPC315.BMWT. 2E6 CAR T cells were injected 3 days later. (F) Schematic of syngeneic antigen-low MOPC315.BMlow in vivo model (used in panels G-I). Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. (G) Tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with IL-18-secreting APRIL or BCMA-CAR T cells. Tumor BLI was performed weekly starting on day 7. Data are combined from at least 2 independent experiments. (H) Tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with BAFF-R-28-1XX CAR T cells ± IL-18. Tumor BLI was performed weekly starting on day 7. (I) Tumor BLI, T-cell BLI, and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with APRIL-BB-1XX/IL-18 T cells compared with control APRIL-BB-1XX T cells without engineered IL-18 expression. T-cell BLI was performed weekly starting on day 6. Tumor BLI was performed weekly starting on day 7. Data are combined from at least 2 independent experiments. (J) Survival of APRIL-BB-1XX/IL-18 treated complete responder mice that were rechallenged with 4E5 MOPC315.BM TACIKO(BCMAlowBAFF-Rlow) 93 days after original experiment start date. Mice were not irradiated before re-challenge. Statistical analysis of all survival curves was performed with the Mantel-Cox log-rank test. ELISA, enzyme-linked immunosorbent assay. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Based on the superior activity of 4-1BB–costimulated APRIL-CAR T cells against antigen-high MOPC315.BMWT, we reasoned that combining 4-1BB costimulation with engineered IL-18 secretion might further promote antimyeloma activity. IL-18 secretion enhanced the activity of APRIL-28BB-1XX T cells, eliminating MOPC315.BMWT in vivo (Figure 2E). Given this improved activity against antigen-high MOPC315.BMWT, we evaluated IL-18–secreting CAR T cells against MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow). We observed long-term survival in nearly all MOPC315.BMlow-bearing mice treated with APRIL-BB-1XX/IL-18, BCMA-28BB-1XX/IL-18, or BAFF-R-28-1XX/IL-18 T cells (Figure 2F-H), demonstrating that IL-18–secreting T cells promote clearance of antigen-low myeloma by targeting either weak BCMA or BAFF-R expression.
Unexpectedly, third-generation APRIL-28BB-1XX/IL-18 T cells induced rapid death in 40% of MOPC315.BMlow-bearing mice, suggesting T-cell–mediated toxicity (Figure 2G, green). APRIL-28BB-1XX/IL-18 T-cell recipients had elevated blood frequency of CAR T cells, compared with the nontoxic APRIL-BB-1XX/IL-18 group (supplemental Figure 6A). Proinflammatory cytokines implicated in cytokine release syndrome (CRS)55-57 were elevated in the serum of APRIL-28BB-1XX/IL-18 T-cell recipients, suggesting CRS-related toxicity (supplemental Figure 6B-C). Interestingly, this toxicity was not observed with APRIL-28BB-1XX/IL-18 T cells in the antigen-high setting (Figure 2E). To investigate the difference between these 2 settings, we performed a 4-day repetitive stimulation of APRIL-28BB-1XX/IL-18 T cells with TACIhigh-MOPC315.BMWT or BCMAlow-MOPC315.BMlow, to simulate sustained T-cell stimulation by myeloma in vivo. APRIL-28BB-1XX/IL-18 T cells strongly upregulated exhaustion–associated inhibitory receptors in response to MOPC315.BMWT, whereas MOPC315.BMlow only stimulated weak upregulation of these molecules (supplemental Figure 6D). Therefore, strong inhibitory receptor upregulation following exposure to antigen-high MOPC315.BMWT may negatively regulate APRIL-28BB-1XX/IL-18 T cells, reducing the risk of CRS-related toxicity. Although APRIL-28BB-1XX/IL-18 T cells generated CRS-associated lethality in MOPC315.BMlow-bearing mice, APRIL-BB-1XX/IL-18 T cells demonstrated strong activity against antigen-low myeloma while also lacking toxicity.
IL-18-secreting 4-1BB-costimulated APRIL-CAR T cells exhibit an effector–differentiated prosurvival transcriptomic signature associated with enhanced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling
To investigate how engineered IL-18 secretion facilitates clearance of antigen-low myeloma, we focused our characterization studies on APRIL-BB-1XX T cells ± engineered IL-18 secretion, due to their in vivo efficacy, lack of toxicity, and ability to target both BCMA and TACI. T-cell BLI demonstrated that engineered IL-18 secretion did not impair the enhanced persistence of APRIL-BB-1XX T cells over several weeks (Figure 2I). Complete responder mice previously treated with APRIL-BB-1XX/IL-18 T cells were protected from rechallenge with MOPC315.BMlow, consistent with durable antimyeloma immunity (Figure 2J).
NF-κB signaling can stimulate T-cell effector function, proliferation, and survival.51,58,59 4-1BB and IL-18 both signal in part through NF-κB51,60-62 and concordantly we measured additive increases in NF-κB reporter activity with 4-1BB costimulation and IL-18 secretion compared with CD28 costimulation (supplemental Figure 7), suggesting that NF-κB signaling may contribute to the persistence and activity of 4-1BB-costimulated IL-18-secreting APRIL-CAR T cells.
To further investigate the enhancement of antimyeloma activity conferred by 4-1BB costimulation combined with IL-18 secretion, we performed single-cell RNA sequencing (scRNA-seq) comparing APRIL-CAR T cells with CD28 or 4-1BB costimulation ± IL-18 coexpression, following antigen stimulation (supplemental Figure 8A). 4-1BB costimulation reduced signatures of T-cell exhaustion compared with CD28 costimulation,48 and combination of 4-1BB/IL-18 retained this phenotype (supplemental Figure 8B-C). Consistent with our previous results, APRIL-BB-1XX/IL-18 T cells upregulated the NF-κB pathway to the greatest extent (supplemental Figure 8D). Compared with APRIL-28-1XX T cells, APRIL-BB-1XX T cells increased both glycolysis and fatty acid metabolism that are associated with T-cell activation and persistence,63,64 while also upregulating survival-associated genes (Stat5, Il21, Il21r) (supplemental Figure 8E-F). APRIL-BB-1XX/IL-18 T cells additionally upregulated genes and pathways related to survival, proliferation, activation, cytotoxicity, and cell adhesion64 (supplemental Figure 8E-G). Some of these genes (Il2, Bcl2, Bach2) are direct targets of NF-κB,65-68 suggesting that increased NF-κB signaling in APRIL-BB-1XX/IL-18 T cells contributes to their enhanced activity. In contrast, APRIL-28-1XX T cells strongly expressed genes encoding exhaustion–associated transcription factors (Tox, Nr4a1/2, Nfatc1) and inhibitory receptors (Pdcd1, Havcr2, Lag3), while IL-18/CD28 further upregulated proapoptotic proteins (Bad, Bid, Bax) (supplemental Figure 8E-G). This suggested that although IL-18 may confer enhanced antimyeloma activity, CD28-driven exhaustion48 and upregulation of apoptosis genes in APRIL-28-1XX/IL-18 T cells might limit activity and persistence in vivo. Our results suggest that 4-1BB costimulation promotes enhanced activation, nutrient metabolism, and survival in APRIL-CAR T cells. Addition of engineered IL-18 secretion to 4-1BB costimulation retains these features while also driving expression of T-cell proliferation and effector function genes.
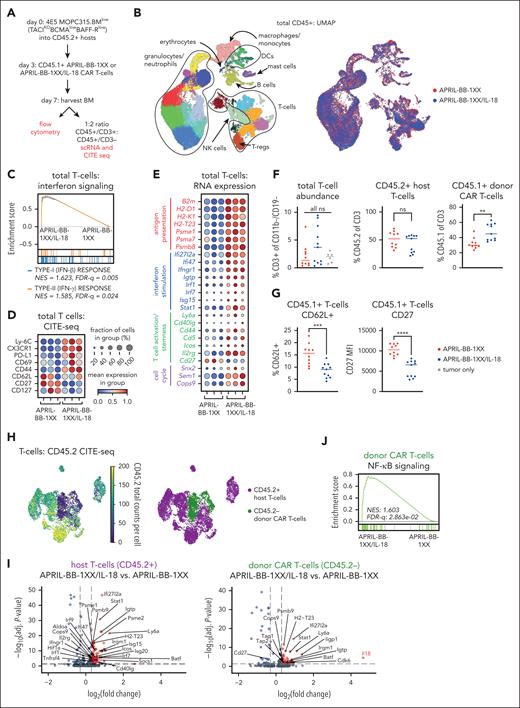
Engineered IL-18 secretion by CAR T cells activates lymphocytes in vivo
To elucidate how engineered IL-18 secretion promotes CAR T-cell activity against MOPC315.BMlow in vivo, we harvested BM of mice 4 days after treatment with APRIL-BB-1XX T cells ± IL-18 (day 7 after tumor) and performed scRNA-seq plus cellular indexing of transcriptomes and epitopes (CITE-seq) on CD45+ cells (Figure 3A-B; supplemental Figure 9). Pathway analysis identified enhanced type-I/II interferon signaling in total T cells from APRIL-BB-1XX/IL-18 T-cell recipients, consistent with IL-18–induced IFN-γ production T cells (Figure 3C).60,69,70 Genes and proteins associated with antigen presentation, interferon responses, T-cell activation, and cell cycle were upregulated in T cells of APRIL-BB-1XX/IL-18 T-cell–treated mice (Figure 3D-E). Several markers associated with naïve T cells (CD62L, CD27, CD127) were downregulated, consistent with IL-18-stimulated effector differentiation. Parallel flow cytometric analysis revealed increased CD45.1+ CAR T cells in the BM of APRIL-BB-1XX/IL-18 T-cell recipients (Figure 3F) and downregulation of CD62L and CD27 expression (Figure 3G).
IL-18-secreting CAR T cells increase interferon signaling and drive effector-like differentiation of T cells in vivo. (A) Experimental setup describing in vivo antigen-low IL-18-CAR T-cell model and BM harvest for flow or single-cell sequencing. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. Harvest data for single-cell sequence or flow cytometry were collected on day 7 of experiment unless otherwise specified. (B) UMAP projection of 34628 CD45+ cells post-QC processing, annotated by different cell types (left) and biological group (right). (C) GSEA plot depicting enrichment of the type-I/II interferon signaling pathways in T cells from APRIL-BB-1XX/IL-18 treated mice. (D-E) Dotplot of selected CITE-seq epitope expression (D) and RNA transcript expression (E) related to antigen presentation, interferon signaling/response, T-cell activation/effector function, T-cell stemness, and cell cycle. Columns represent 3 replicate mice in each group. (F) Abundance of total T cells, CD45.2+ host T cells, and CD45.1+ donor CAR T cells, as measured by flow cytometry. Data are combined from at least 2 independent experiments. Statistics performed with the 1-way ANOVA test. (G) Expression of stemness/naïve-like markers in CD45.1+ donor CAR T cells, as measured by flow cytometry. Data are combined from at least 2 independent experiments. (H) UMAP projection of T cells and annotation of CD45.2+ host T cells and CD45.2– donor CAR T cells. Annotations were assigned using CD45.2 expression with CITE-seq. (I) Volcano plots of the Wilcoxon rank-sum scores from the differential gene expression analysis of APRIL-BB-1XX/IL-18 vs APRIL-BB-1XX CAR T-cell treatment in CD45.2+ host T cells and CD45.2– donor CAR T cells, respectively. Genes with adjusted P value = 1 were filtered out before plotting. (J) GSEA plot displaying upregulation of NF-κB signaling pathway in CD45.2– donor APRIL-BB-1XX/IL-18 T cells. UMAP: Uniform Manufold Approximation and Projection; GSEA: gene set enrichment analysis. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
IL-18-secreting CAR T cells increase interferon signaling and drive effector-like differentiation of T cells in vivo. (A) Experimental setup describing in vivo antigen-low IL-18-CAR T-cell model and BM harvest for flow or single-cell sequencing. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. Harvest data for single-cell sequence or flow cytometry were collected on day 7 of experiment unless otherwise specified. (B) UMAP projection of 34628 CD45+ cells post-QC processing, annotated by different cell types (left) and biological group (right). (C) GSEA plot depicting enrichment of the type-I/II interferon signaling pathways in T cells from APRIL-BB-1XX/IL-18 treated mice. (D-E) Dotplot of selected CITE-seq epitope expression (D) and RNA transcript expression (E) related to antigen presentation, interferon signaling/response, T-cell activation/effector function, T-cell stemness, and cell cycle. Columns represent 3 replicate mice in each group. (F) Abundance of total T cells, CD45.2+ host T cells, and CD45.1+ donor CAR T cells, as measured by flow cytometry. Data are combined from at least 2 independent experiments. Statistics performed with the 1-way ANOVA test. (G) Expression of stemness/naïve-like markers in CD45.1+ donor CAR T cells, as measured by flow cytometry. Data are combined from at least 2 independent experiments. (H) UMAP projection of T cells and annotation of CD45.2+ host T cells and CD45.2– donor CAR T cells. Annotations were assigned using CD45.2 expression with CITE-seq. (I) Volcano plots of the Wilcoxon rank-sum scores from the differential gene expression analysis of APRIL-BB-1XX/IL-18 vs APRIL-BB-1XX CAR T-cell treatment in CD45.2+ host T cells and CD45.2– donor CAR T cells, respectively. Genes with adjusted P value = 1 were filtered out before plotting. (J) GSEA plot displaying upregulation of NF-κB signaling pathway in CD45.2– donor APRIL-BB-1XX/IL-18 T cells. UMAP: Uniform Manufold Approximation and Projection; GSEA: gene set enrichment analysis. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To distinguish CD45.2+ host from CD45.2– donor (CAR) T cells, we assessed CD45.2 CITE-seq epitope expression and assigned host/donor annotations (Figure 3H). Both CD45.2+ host and CD45.2– donor T cells upregulated genes related to interferon stimulation, antigen presentation, and T-cell activation in APRIL-BB-1XX/IL-18 T-cell recipients (Figure 3I). We observed strong upregulation of Il18, downregulation of Cd27, and again confirmed increased NF-κB pathway activation in CD45.2– donor APRIL-BB-1XX/IL-18 T cells (Figure 3I-J) suggesting that IL-18-secreting APRIL-BB-1XX T cells induced an effector/differentiated T-cell state,71-73 but did not impair persistence in vivo (Figure 2I), possibly due to the prosurvival gene expression conferred by 4-1BB costimulation (supplemental Figure 8E-F). Our results demonstrate that treatment with APRIL-BB-1XX/IL-18 T cells enhanced activation, effector differentiation, and interferon response in all T cells, consistent with proinflammatory reprogramming of the myeloma microenvironment.
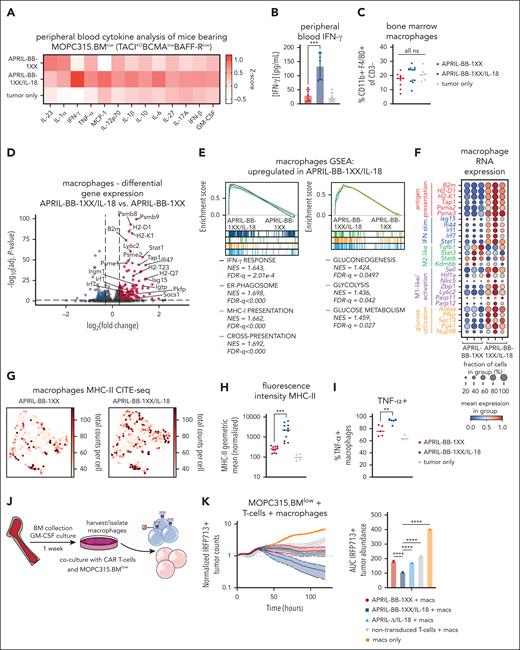
Stimulation of macrophages by IL-18-secreting CAR T cells promotes killing of antigen-low myeloma
CAR T cells can lyse malignant cells directly or activate endogenous immune cells to promote antitumor activity.74 Although APRIL-BB-1XX/IL-18 T cells effectively cleared MOPC315.BMlow in vivo, APRIL-BB-1XX T cells failed to lyse these cells effectively in vitro, even with engineered IL-18 secretion (supplemental Figure 10A-B). Our experiments with APRIL-Δ/IL-18 and CD19-CAR/IL-18 T cells demonstrated that CAR signaling was required for IL-18–mediated antimyeloma activity (Figure 2D; supplemental Figure 5E), suggesting that APRIL-BB-1XX/IL-18 T cells recognize weak BCMA expression on MOPC315.BMlow. Indeed, APRIL-BB-1XX/IL-18 T cells responded to MOPC315.BMlow by upregulating engineered IL-18 secretion (supplemental Figure 10C), indicating weak BCMA expression could stimulate CAR–mediated viral vector expression.75,76 Therefore, we postulated the activity of IL-18-secreting CAR T cells against antigen-low myeloma might depend on stimulation of host effector cells through CAR-mediated promotion of IL-18 secretion, rather than direct T-cell cytotoxicity.
Previous studies established that IFN-γ-stimulated macrophages play a critical role for IL-18-CAR T-cell efficacy in lymphoma17 and for idiotype-specific CD4+ T-cell–mediated elimination of MOPC315.BM.77,78 IL-18 induced NF-κB signaling60-62 can also activate the Ifng promoter.79,80 We observed increased IFN-γ in serum of APRIL-BB-1XX/IL-18 T-cell recipients (Figure 4A-B), consistent with upregulation of interferon pathways observed in BM T cells (Figure 3). Although BM macrophage abundance remained unchanged (Figure 4C; supplemental Figure 9E), scRNA-seq analysis of macrophages in APRIL-BB-1XX/IL-18 T-cell–treated mice revealed upregulation of genes and pathways related to interferon signaling, antigen presentation, and glycolysis (Figure 4D-E), reflecting a proinflammatory, antitumor M1-like phenotype.81-83 We also identified M1-associated genes trending up and anti-inflammatory M2–associated genes trending down in macrophages of APRIL-BB-1XX/IL-18 T-cell recipients (Figure 4F).84,85 We validated major histocompatibility complex-II surface upregulation with CITE-seq and flow cytometry, and additionally measured more tumor necrosis factor-α expressing macrophages from APRIL-BB-1XX/IL-18 T-cell recipients (Figure 4G-I). Dendritic cell (DC) abundance increased in recipients of APRIL-BB-1XX/IL-18 T cells and skewed toward a proinflammatory cDC1 phenotype, with upregulation of genes associated with antigen presentation and interferon signaling (supplemental Figure 11A).86 Additionally, these DCs displayed increased transcripts related to cDC1–associated protein production and mitochondrial oxidative phosphorylation (supplemental Figure 11B-C).87
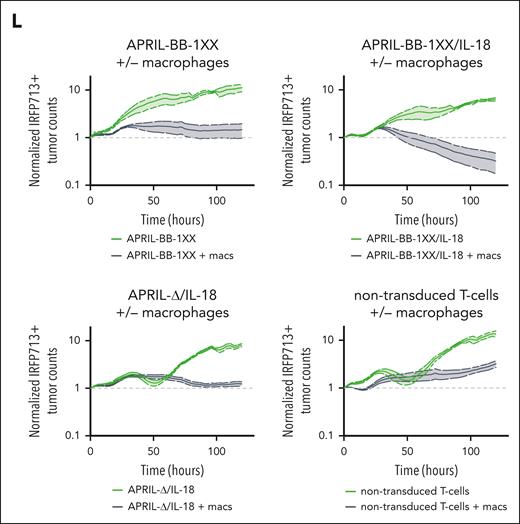
To interrogate the functional effect of macrophages in eradication of MOPC315.BMlow with IL-18–secreting CAR T cells, we differentiated F4/80+ macrophages88,89 from naïve mice and cocultured them with APRIL-CAR T cells and MOPC315.BMlow (Figure 4J; supplemental Figure 12A). Although APRIL-BB-1XX/IL-18 T cells previously failed to kill antigen-low myeloma in vitro (supplemental Figure 10C), addition of macrophages with CAR T cells resulted in target lysis (Figure 4K-L). APRIL-BB-1XX and APRIL-Δ/IL-18 T cells cultured with macrophages also exerted weak activity against MOPC315.BMlow, reflecting the requirement for combined CAR signaling and IL-18 secretion to generate activity in vivo (Figure 2D). Addition of both exogenous recombinant murine-IL-18 and macrophages similarly licensed antimyeloma activity of APRIL-BB-1XX T cells without engineered IL-18 secretion (supplemental Figure 12B). However, exogenous IL-18 and macrophages alone were unable to eliminate MOPC315.BMlow in the absence of CAR T cells. These results indicate that IL-18–secreting CAR T cells promote macrophage-mediated clearance of MOPC315.BMlow.
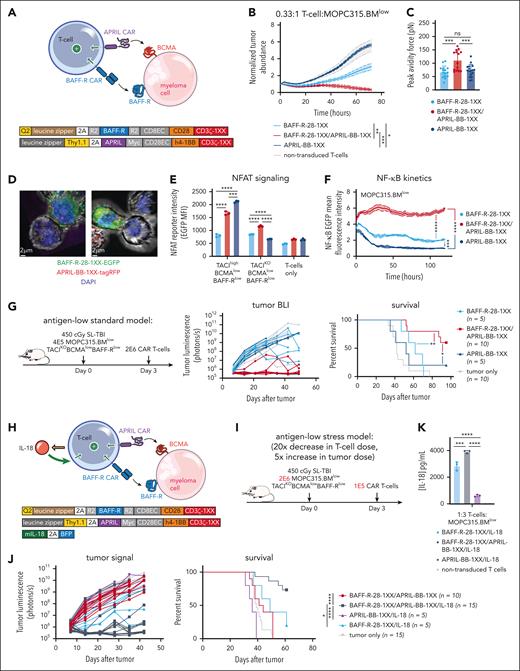
BAFF-R/APRIL dual-CAR T cells with engineered IL-18 secretion eliminate antigen-low myeloma in a stress-dose model
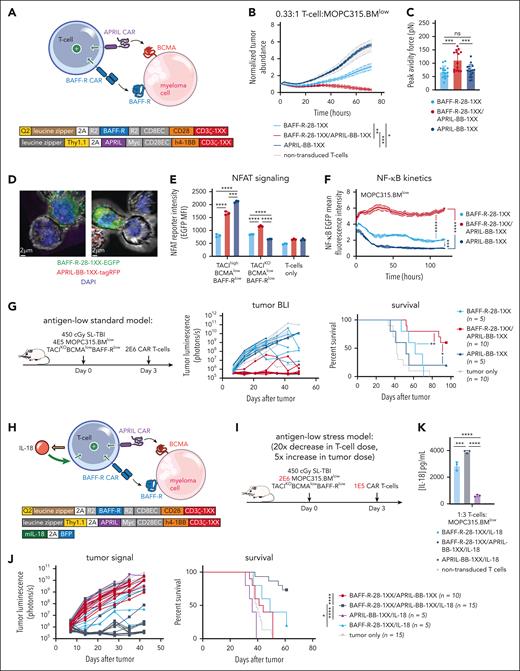
While APRIL-BB-1XX T cells were unable to kill MOPC315.BMlow directly, we reasoned that a dual-CAR approach might promote cytotoxic T-cell killing of malignant cells by enabling combined CAR stimulation by 2 weakly-expressed antigens. We further hypothesized that combining the APRIL ligand–based CAR (which binds BCMA + TACI) and the BAFF-R CAR, in conjunction with IL-18 secretion might further increase activity by promoting stronger CAR signaling, target killing, and IL-18 output. We first paired BAFF-R-28-1XX with APRIL-BB-1XX to target weak BAFF-R and BCMA expression coordinately (Figure 5A). BAFF-R/APRIL dual-CAR T cells demonstrated enhanced lytic activity in vitro against MOPC315.BMlow (Figure 5B). Dual-CAR expression produced higher T-cell:MOPC315.BMlow cellular avidity, consistent with tighter cell binding improving lytic activity (Figure 5C).90 Confocal microscopy confirmed that enhanced green fluorescence protein–labeled BAFF-R-28-1XX and tag-RFP–labeled APRIL-BB-1XX colocalized at the T-cell synaptic interface with MOPC315.BMlow (Figure 5D). Dual-CAR T cells exhibited increased nuclear factor of activated T cells and sustained NF-κB signaling when exposed to MOPC315.BMlow (Figure 5E-F). Consistent with improved in vitro lysis, BAFF-R/APRIL dual-CAR T cells achieved long-term survival in 60% of MOPC315.BMlow-bearing mice (Figure 5G). Together, these findings suggested that increased cellular avidity and CAR signal strength resulting from targeting 2 weakly-expressed antigens promoted T-cell killing of antigen-low MOPC315.BMlow.
BAFF-R/APRIL dual-CAR T cells with engineered IL-18 secretion eliminate antigen-low myeloma in a stress-dose model. (A) Schematic of BAFF-R/APRIL dual-CAR T cells and the corresponding CAR vector maps. (B) Incucyte cytotoxicity assay of BAFF-R/APRIL dual or single-CAR T cells vs 10 000 IRFP713+ MOPC315.BMlow. Near-infrared signal was measured over time with the Incucyte SX5. Statistical analysis was performed with the 1-way ANOVA test at 72-hour end point. Data are representative of at least 2 independent experiments. (C) Binding avidity force between MOPC315.BMlow and BAFF-R/APRIL dual or single-CAR T cells. Myeloma cells were seeded on poly-L-lysine coated plates before adding EGFP+ T cells. Lumicks C-trap optical tweezers were used to locate EGFP+ T cells, bring them in contact with myeloma cells, and then separate them, allowing separating force to be recorded. Statistical analysis was performed with the 1-way ANOVA test. Data are combined from at least 2 independent experiments. (D) Confocal microscopy of coexpressed BAFF-R-EGFP and APRIL-tagRFP CARs present at the synapse interface between CAR T cells and MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow). CAR T cells were cocultured with MOPC315.BMlow for 2 hours and then fixed and processed for imaging. Z-stack images were acquired with Leica Stellaris 8 at 63× magnification and room temperature (25°C), in the following channels: green, red, and DAPI. Data were analyzed on the Imaris software (Oxford Instruments) and 2D images containing a clear representation of the T-cell:MOPC315.BMlow synaptic interface were exported. (E) NFAT signaling of BAFF-R/APRIL dual or single-CAR T cells when exposed MOPC315.BMWT or MOPC315.BMlow myeloma cells. A total of 20 000 CAR T cells containing an NFAT-EGFP response element were cocultured with 60 000 myeloma cells for 24 hours. NFAT-GFP signaling was measured with flow cytometry. Data are representative of at least 2 independent experiments. (F) NF-κB signaling of BAFF-R/APRIL dual or single-CAR T cells. 10 000 CAR T cells containing an NF-κB-EGFP response element were cocultured with 30 000 MOPC315.BMlow cells. NF-κB-EGFP signal was measured over time with the Incucyte SX5. Statistical analysis was performed with the 1-way ANOVA test at 120 hours. Data are representative of at least 2 independent experiments. (G) Antigen-low myeloma standard dosing schematic, tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with BAFF-R/APRIL dual or single APRIL or BAFF-R-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. Statistical analysis of survival was performed with the Mantle-Cox log-rank test. Data are combined from at least 2 independent experiments. (H) Schematic of IL-18-secreting BAFF-R/APRIL dual-CAR T cells and the corresponding vector maps. (I) Model setup of stress-dose model of MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) with a 5× increase in tumor dose and 20× decrease in T-cell dose. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E6 MOPC315.BMlow. 1E5 CAR T cells were injected 3 days later. (J) Tumor BLI and survival of mice bearing 2E6 MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with a low dose of 1E5 IL-18-secreting BAFF-R/APRIL dual-CAR T cells, BAFF-R/APRIL dual-CAR T cells alone, BAFF-R/IL-18 CAR T cells, or APRIL/IL-18 CAR T cells. Statistical analysis of survival was performed with the Mantel-Cox log-rank test. Data are combined from at least 3 independent experiments. (K) ELISA of IL-18 production from BAFF-R-28-1XX/APRIL-BB-1XX/IL-18 compared with BAFF-R-28-1XX/IL-18 and APRIL-BB-1XX/IL-18 CAR T cells when cocultured with MOPC315.BMlow. Statistical analysis was performed with the 1-way ANOVA test. DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
BAFF-R/APRIL dual-CAR T cells with engineered IL-18 secretion eliminate antigen-low myeloma in a stress-dose model. (A) Schematic of BAFF-R/APRIL dual-CAR T cells and the corresponding CAR vector maps. (B) Incucyte cytotoxicity assay of BAFF-R/APRIL dual or single-CAR T cells vs 10 000 IRFP713+ MOPC315.BMlow. Near-infrared signal was measured over time with the Incucyte SX5. Statistical analysis was performed with the 1-way ANOVA test at 72-hour end point. Data are representative of at least 2 independent experiments. (C) Binding avidity force between MOPC315.BMlow and BAFF-R/APRIL dual or single-CAR T cells. Myeloma cells were seeded on poly-L-lysine coated plates before adding EGFP+ T cells. Lumicks C-trap optical tweezers were used to locate EGFP+ T cells, bring them in contact with myeloma cells, and then separate them, allowing separating force to be recorded. Statistical analysis was performed with the 1-way ANOVA test. Data are combined from at least 2 independent experiments. (D) Confocal microscopy of coexpressed BAFF-R-EGFP and APRIL-tagRFP CARs present at the synapse interface between CAR T cells and MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow). CAR T cells were cocultured with MOPC315.BMlow for 2 hours and then fixed and processed for imaging. Z-stack images were acquired with Leica Stellaris 8 at 63× magnification and room temperature (25°C), in the following channels: green, red, and DAPI. Data were analyzed on the Imaris software (Oxford Instruments) and 2D images containing a clear representation of the T-cell:MOPC315.BMlow synaptic interface were exported. (E) NFAT signaling of BAFF-R/APRIL dual or single-CAR T cells when exposed MOPC315.BMWT or MOPC315.BMlow myeloma cells. A total of 20 000 CAR T cells containing an NFAT-EGFP response element were cocultured with 60 000 myeloma cells for 24 hours. NFAT-GFP signaling was measured with flow cytometry. Data are representative of at least 2 independent experiments. (F) NF-κB signaling of BAFF-R/APRIL dual or single-CAR T cells. 10 000 CAR T cells containing an NF-κB-EGFP response element were cocultured with 30 000 MOPC315.BMlow cells. NF-κB-EGFP signal was measured over time with the Incucyte SX5. Statistical analysis was performed with the 1-way ANOVA test at 120 hours. Data are representative of at least 2 independent experiments. (G) Antigen-low myeloma standard dosing schematic, tumor BLI and survival of mice bearing MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with BAFF-R/APRIL dual or single APRIL or BAFF-R-CAR T cells. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 4E5 MOPC315.BMlow. 2E6 CAR T cells were injected 3 days later. Statistical analysis of survival was performed with the Mantle-Cox log-rank test. Data are combined from at least 2 independent experiments. (H) Schematic of IL-18-secreting BAFF-R/APRIL dual-CAR T cells and the corresponding vector maps. (I) Model setup of stress-dose model of MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) with a 5× increase in tumor dose and 20× decrease in T-cell dose. Mice were lymphodepleted with 450 cGy SL-TBI on day 0 and then injected tail IV with 2E6 MOPC315.BMlow. 1E5 CAR T cells were injected 3 days later. (J) Tumor BLI and survival of mice bearing 2E6 MOPC315.BMlow (TACIKOBCMAlowBAFF-Rlow) and treated with a low dose of 1E5 IL-18-secreting BAFF-R/APRIL dual-CAR T cells, BAFF-R/APRIL dual-CAR T cells alone, BAFF-R/IL-18 CAR T cells, or APRIL/IL-18 CAR T cells. Statistical analysis of survival was performed with the Mantel-Cox log-rank test. Data are combined from at least 3 independent experiments. (K) ELISA of IL-18 production from BAFF-R-28-1XX/APRIL-BB-1XX/IL-18 compared with BAFF-R-28-1XX/IL-18 and APRIL-BB-1XX/IL-18 CAR T cells when cocultured with MOPC315.BMlow. Statistical analysis was performed with the 1-way ANOVA test. DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Finally, to evaluate the therapeutic potential of combining dual-CAR targeting and engineered IL-18 secretion, we coexpressed an IL-18-encoding vector (IL-18- blue fluorescence protein) along with BAFF-R-28-1XX/APRIL-BB-1XX in T cells and evaluated them in a stress-dose model of MOPC315.BMlow to enable discrimination of potentially enhanced T-cell activity against a larger myeloma disease burden (Figure 5H). We increased the tumor burden 5× to 2E6/mouse and reduced the CAR T-cell dose 20× to 1E5/mouse (Figure 5I). In this setting, BAFF-R-28-1XX/APRIL-BB-1XX/IL-18 T cells eliminated antigen-low myeloma in >70% of mice, while BAFF-R-28-1XX/APRIL-BB-1XX, BAFF-R-28-1XX/IL-18, or APRIL-BB-1XX/IL-18 T cells failed to control disease (Figure 5J). Compared with BAFF-R-28-1XX/IL-18 or APRIL-BB-1XX/IL-18 T cells, BAFF-R-28-1XX/APRIL-BB-1XX/IL-18 dual-CAR T cells secreted more IL-18 in response to MOPC315.BMlow, consistent with greater CAR–induced transgene activation (Figure 5K). Our results demonstrate that dual-targeting of 2 weakly-expressed antigens with a BAFF-R/APRIL dual-CAR increases T-cell myeloma avidity, enhances T-cell signaling, and promotes survival in mice. Coexpression of IL-18 with this dual-CAR promotes clearance of antigen-low myeloma at low T-cell doses and is characterized by increased IL-18 output.
Discussion
Although BCMA–directed CAR T cells have achieved impressive responses in myeloma patients, therapeutic failure can occur due to weak or absent antigen expression, insufficient CAR binding to target, or suboptimal activation of T cells.11,13,15,91 Multiantigen targeting,19 enhancement of T-cell activity with optimal costimulation,20,22 and increasing CAR sensitivity to target antigens24 have been investigated preclinically against antigen-low disease but are not yet widespread clinically. Ciltacabtagene autoleucel implements 2 distinct BCMA–targeting variable heavy chain domains which may increase T-cell:myeloma avidity.92 This approach appears promising, as the CARTITUDE-4 trial (NCT04181827) reported that ciltacabtagene autoleucel had not reached median progression free survival at 27 months.10 Using a syngeneic mouse model, we employed 2 strategies to address antigen-low myeloma: engineered IL-18 secretion to stimulate endogenous immune cells, and simultaneous targeting of weak BCMA and BAFF-R to increase overall T-cell activation. Both approaches were successful independently and combination of the 2 potentiated therapy for larger antigen-low myeloma burdens.
The antimyeloma activity of IL-18-secreting CAR T cells required CAR signaling and was characterized by T-cell and myeloid-cell activation in the BM microenvironment of myeloma, despite inability of APRIL-BB-1XX/IL-18 T cells to kill MOPC315.BMlow in vitro. Recognition of weak BCMA expression by APRIL-BB-1XX increased T-cell secretion of engineered IL-18. APRIL-BB-1XX/IL-18 T cells resulted in elevated serum IFN-γ levels,60,61,79,80 and enhanced type-I/II interferon signaling in T cells and myeloid cells. This proinflammatory environment polarized macrophages toward an M1-like state,81,83,93,94 and macrophages were required for elimination of MOPC315.BMlow.17,70,77,78
APRIL-BB-1XX T cells demonstrated superior antitumor activity and persistence against antigen-high myeloma compared with APRIL-28-1XX T cells, and APRIL-BB-1XX/IL-18 coexpression increased activity against antigen-low myeloma without toxicity. 4-1BB costimulation promoted T-cell persistence and transcriptomic signatures associated with enhanced survival and reduced exhaustion. Addition of IL-18 maintained these features and further stimulated NF-κB–associated effector differentiation and proliferation. This suggests that combination of 4-1BB costimulation with IL-18 secretion in CAR T cells may promote maintenance of T-cell effector function and consequent IFN-γ–mediated reprogramming of cells in the myeloma microenvironment to eliminate antigen-low disease.
APRIL-28BB-1XX/IL-18 T cells produced lethal toxicity in mice bearing antigen-low, but not antigen-high myeloma, possibly due to inhibitory receptor upregulation upon stimulation with antigen-high targets. This toxicity was characterized by increased APRIL-28BB-1XX/IL-18 T-cell expansion and elevated CRS-related cytokines55,57 in BCMAlow-MOPC315.BMlow-bearing mice. Though we did not observe this toxicity with other CAR/IL-18 configurations, our data cautions that stronger costimulation combined with IL-18 secretion in T cells can induce CRS-associated lethality. However, we also did not observe toxicity with dose-reduced IL-18-secreting dual-CAR T cells, which have both CD28/4-1BB costimulation on separate CARs and maintained efficacy in vivo. Low doses of IL-18–secreting CD19-CAR T cells have generated complete responses in patients,40 suggesting dose optimization of potent T-cell therapies may establish nontoxic therapeutic windows.
Despite the efficacy of APRIL-CD28-OX40-CD3ζ T cells in preclinical studies,36 these cells demonstrated poor activity in patients (NCT03287804).15 This APRIL-CAR exhibited insufficient BCMA-binding and weak T-cell myeloma avidity interactions compared with the clinically approved scFvs, leading to diminished antimyeloma activity following repeated stimulation. Trimeric APRIL-CAR T cells exhibited improved binding to myeloma targets,42 and our data revealed that combining the APRIL-CAR with a second CAR (BAFF-R) increased T-cell:myeloma avidity, enhancing T-cell activation. Additionally, we demonstrated that IL-18–secreting CAR T cells activated macrophages to mediate antimyeloma activity, providing an alternate mechanism for poorly binding or signaling CAR T cells to eradicate antigen-low disease. Recent data indicate BCMA extracellular domain mutations prevent binding of clinically approved BCMA-directed therapies.95 APRIL retained binding capacity despite these mutations, suggesting APRIL may remain a viable antigen-binding domain candidate, especially when combined with IL-18 secretion. APRIL targets TACI as an alternative antigen when BCMA is insufficiently expressed or mutated to subvert binding. Additionally, the BAFF-R/APRIL dual-CAR can theoretically target BCMA, TACI, or BAFF-R simultaneously, providing 3 options for recognition of myeloma.
In conclusion, we demonstrate that engineered IL-18 secretion and multiantigen targeting promote clearance of antigen-low multiple myeloma via distinct mechanisms that can stimulate T cells or myeloid cells. Engineered IL-18 secretion enabled single APRIL, BCMA, or BAFF-R-CAR T cells to individually eradicate antigen-low myeloma, which was associated with inflammatory reprogramming of the myeloma microenvironment. BAFF-R/APRIL dual-CAR T cells targeting weakly-expressed BCMA and BAFF-R increased T-cell:myeloma cellular avidity and enhanced T-cell signaling that promoted myeloma lysis. Coexpression of IL-18 in BAFF-R/APRIL dual-CAR T cells further potentiated antimyeloma activity. Because many tumors exhibit variable antigen expression74 and activation of macrophages can promote tumor clearance in multiple cancer models,17,77,96 combination of IL-18 secretion with multiantigen targeting may represent a strategy to improve CAR T-cell activity in various malignancies.
Acknowledgments
The authors thank the Memorial Sloan Kettering Cancer Center (MSKCC) Single-cell Analytics Innovation Lab, led by Ronan Chaligne, for assistance with planning the single-cell sequencing experiment, droplet formation, library preparation, sequencing of cells, and processing of FASTQ files after submission; Teng Fei of the van den Brink laboratory and the Department of Epidemiology and Biostatistics at MSKCC for providing advice with statistical analysis; and Renier Brentjens at Roswell Park Comprehensive Cancer Center who is a member of B.D.N.’s thesis committee and provided early guidance on using engineered IL-18 secretion.
This research was supported by National Cancer Institute (NCI), National Institutes of Health (NIH), awards R35-CA284024, P01-CA023766, R01-CA228308, and P30-CA008748 MSK Cancer Center Support Grant/Core Grant; National Heart, Lung, and Blood Institute, NIH, awards R01-HL164902 and R01 HL147584; National Institute on Aging, NIH, award P01-AG052359, and the Tri-Institutional Stem Cell Initiative. Additional funding was received from The Lymphoma Foundation, The Susan and Peter Solomon Family Fund, The Solomon Microbiome Nutrition and Cancer Program, Cycle for Survival, Parker Institute for Cancer Immunotherapy, Paula and Rodger Riney Multiple Myeloma Research Initiative, Starr Cancer Consortium, and Seres Therapeutics. S.C. was supported by a research fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft). H.K.E. is supported by a grant from the Parker Institute for Cancer Immunotherapy. A.P.B. is supported by the Lymphoma Research Foundation lymphoma scientific research mentoring program grant and a Young Investigator Award from the American Society of Clinical Oncology. A.L.L. is supported by the Sweden-America Foundation, Svenska Sällskapet för Medicinsk Forskning and Deutsche Knochenmarkspenderdatei. S.D. receives research support from the MSK Leukemia SPORE Career Enhancement Program (NCI/NIH) P50 CA254838-01) and the Gerstner Physician Scholar program. S.E.J. is supported by a K08 career development award (NCI/NIH) K08-CA252157, a Young Investigator award from the American Society of Clinical Oncology, an Amy Program Award from the Be The Match Foundation, and a Bridge Scholar award from the Parker Institute for Cancer Immunotherapy.
Authorship
Contribution: B.D.N., S.E.J., and M.R.M.v.d.B. developed the study; B.D.N. and S.E.J. designed vectors and experiments; B.D.N. performed the primary experiments, analyzed all data, and wrote the manuscript; A.R., J.S.F., S.C., A.M., H.K.E., D.M., A.P.B., S.D., and J.P. assisted with development and execution of select experiments; A.I.K. provided pipelines and code for single-cell data analysis; A.I.K. and A.L.L. provided intellectual guidance regarding single-cell data analysis techniques; M.G. provided planning guidance, technical expertise, and instrument control for C-trap and confocal microscopy experiments; B.B. provided the cell line MOPC315.BM and insight on model behavior; and all authors reviewed the manuscript and optionally provided comments or suggested edits before submission.
Conflict-of-interest disclosure: A.P.B. has received compensation for participating in consulting activities with Bristol Myers Squibb. S.E.J. and M.R.M.v.d.B. are coinventors on patent applications related to this work. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaskoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (spouse), Synthekine (spouse), BeiGene (spouse), Kite (spouse); has intellectual property licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the foundation board of Deutsche Knochenmarkspenderdatei (a nonprofit organization). Memorial Sloan Kettering Cancer Center has institutional financial interests relative to Seres Therapeutics. B.B. is leader of the scientific advisory board in the Nykode Therapeutics (former Vaccibody AS) company in which he holds shares. The remaining authors declare no competing financial interests.
Correspondence: Marcel R. M. van den Brink, City of Hope, 1500 East Duarte Rd., Duarte, CA 91010; email: mvandenbrink@coh.org; and Scott E. James, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: jamess2@mskcc.org.
References
Author notes
S.E.J. and M.R.M.v.d.B. cosupervised and contributed equally to this study.
Jupyter notebooks describing data quality control and analysis of single-cell sequencing data will be uploaded to GitHub, whereas raw and processed data have been uploaded to the Gene Expression Omnibus database (accession numbers GSE246682 and GSE261852).
Original data are available on request from the corresponding authors, Marcel R. M. van den Brink (mvandenbrink@coh.org) and Scott E. James (jamess2@mskcc.org).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.