In this issue of Blood, Nie et al report restored antitumor immunity in patients with relapsed/refractory (R/R) classical Hodgkin lymphoma (HL) with an epi-immunotherapy consisting of chidamide, decitabine, and the anti-PD-1 antibody camrelizumab.1 Importantly, significant efficacy was observed in patients relapsing after previous anti–PD-1-based combination treatment. Moreover, the authors identify correlates of response and resistance through comprehensive single-cell analyses of longitudinal HL biopsies.1
Objective response rates (ORRs) to immune checkpoint inhibition (ICI) with the Food and Drug Administration–approved anti-PD-1 antibodies nivolumab and pembrolizumab in R/R HL are high (71%). Complete remission rates (CRRs), however, are only 21% to 28%, and median progression-free survival (mPFS) is 13.7 to 15.1 months. Most patients with R/R HL will eventually fail antiPD1 ICI with a 5-year PFS of only 14% to 18%.2,3 To address the unmet need of anti-PD-1 failure, various strategies such as adding conventional chemotherapy (eg, gemcitabine),4 localized radiotherapy,5 or the histone deacetylase inhibitor (HDACi) vorinostat6 to anti-PD-1 antibodies have been explored. Nie et al previously reported efficacy for the combination of the hypomethylating agent decitabine at low dose (10 mg/d, days 1-5, every 3 weeks [Q3W]) and the anti-PD-1 antibody camrelizumab (200 mg day 8, Q3W) in R/R HL after anti-PD-1 in a phase 2 trial.7 Indeed, the potential to overcome anti-PD-1 failure with decitabine + anti-PD-1 (DP) was confirmed in an expansion cohort (ORR: 52%, CRR: 36%). The majority of patients, however, eventually relapsed, requiring additional treatment.8
Herein, the authors report data from a phase 2 trial (NCT04233294) investigating the addition of the HDACi chidamide (10 mg/d, days 1-4 and 20 mg/d on days 8, 11, 15, 18, Q3W) to DP in 52 patients after failure of DP. Importantly, although the majority of patients (81%) had progressive R/R HL during DP as their last line of therapy, a very high ORR (94%; 95% confidence interval [CI]: 84%-99%) and CRR (50%; 95% CI: 36%-64%) were observed with HDACi chidamide + DP (CDP). The resulting mPFS was 29.4 months, and 1- and 2-year PFS rates were 94% (95% CI: 88%-100%) and 60% (95% CI: 45%-74%), respectively, with a 100% overall survival rate at a median follow-up of 32 months. Notably, the 1-year relapse-free survival was 78% among the 12 patients who discontinued CDP treatment after achieving a sustained CR. With responses observed in all 12 patients that did not respond to prior DP treatment and what seems to be improved mPFS (CDP 26.1 months, DP 11.5 months, P 8.0 months in different lines of treatment), CDP indeed appears to be able to overcome resistance to anti-PD-1 blockade in the majority of patients with R/R HL while being quite well tolerated.
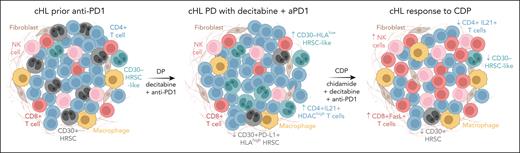
In addition to these encouraging clinical observations, the authors performed 10× 5′ single-cell RNA sequencing (scRNAseq) of 17 lymph node biopsies from 9 patients, obtained either before and/or after treatment with DP and CDP. Including and analyzing an additional 7 HL biopsies, they comprise a comprehensive atlas of 185,791 single-cell transcriptomes and 82 684 T-cell receptor clonotypes from HL biopsies. Bioinformatically leveraging this unique resource in combination with multiplex immunofluorescence, the authors identify and characterize a CD30− Hodgkin and Reed-Sternberg cell (HRSC)-like population. Interestingly, this CD30− HRSC-like population exhibits higher differentiation potential but lower pathway activity of antigen processing and presentation as well as lower CD274 expression, hinting at a developmental trajectory from these atypical cells toward CD30+ HRSC. Although the CD30+ HRSC-like population is effectively depleted by both DP and CDP treatment, the CD30− HRSC-like population is found in DP-resistant tumors but is eradicated after CDP exposure. Further exploring tumor-cell intrinsic determinants of antitumor immunity in the context of epi-immunotherapy, the authors identify an upregulation of HLA gene expression after exposure to epigenetic agents, resulting in an increased conjugation of the HL cell line L1236 and healthy peripheral blood mononuclear cells in in vitro assays.
Although previous analyses failed to identify relevant expansion of cytotoxic CD8+ T cells in the context of anti-PD-1 blockade in HL,9,10 CDP resulted in clonal expansion of CD8+ T cells and appeared to alleviate T-cell exhaustion. Interestingly, some of these populations were already present prior to initiation of CDP (eg, HAVCR2+ T cells), which the authors interpret as successful T-cell activation but lack of antitumor execution with prior anti-PD-1-based treatments. After CDP treatment, a FASL/FAS interaction of CD8+ T cells and natural killer cells with CD30− HRSC-like cells emerged, indicating cytotoxic effector cell activation. Among CD4+ T-cell clusters, Nie et al identify different IL21+ populations with coexpression of T-follicular-helper cell markers. Based on cellular interaction analysis of scRNAseq data, these IL21+ CD4+ T cells appear to form a positive feedback loop with CD30+ HRSC-like cells. The apparent resistance to DP associated with these IL21+ CD4+ T cells is overcome by CDP, and interestingly, these cells show increased expression of HDAC1 core complex genes (HDAC score), which was associated with response to CDP. Of note, HDAC score was low in the CD30− HRSC-like population. Taken together, this is indicative of an indirect therapeutic effect via remodeling of the tumor microenvironment (TME) and upregulation of major histocompatibility complex molecules, thereby enabling an active (and sustained?) anti-HL immune response through dual epigenetic targeting with anti-PD-1 (see figure).
Schematic depiction of the proposed mechanisms of anti-PD-1 failure and efficacy of dual epigenetic targeting in R/R cHL. Simplified schematic depiction of selected key correlates of failure with DP as well as response to CDP treatment or R/R HL reported in the study by Nie and coauthors. cHL, classical Hodgkin lymphoma; FasL, Fas ligand; NK natural killer; PD-L1, programmed death protein 1 ligand 1.
Schematic depiction of the proposed mechanisms of anti-PD-1 failure and efficacy of dual epigenetic targeting in R/R cHL. Simplified schematic depiction of selected key correlates of failure with DP as well as response to CDP treatment or R/R HL reported in the study by Nie and coauthors. cHL, classical Hodgkin lymphoma; FasL, Fas ligand; NK natural killer; PD-L1, programmed death protein 1 ligand 1.
From a clinical perspective, the present study is limited by its single-center design and lack of previous exposure to brentuximab vedotin and/or autologous stem-cell transplantation in most patients. The results are nevertheless impressive and address a key unmet need in R/R HL. They clearly indicate a role for epigenetic modifiers in combination with anti-PD-1 blockade to successfully treat patients with HL by introducing an epi(geneti)c TME makeover, allowing eradication of persisting HRSC populations. To this end, results of an ongoing randomized trial (NCT04514081) of DP vs CDP will be important to more clearly estimate the benefits of dual epigenetic targeting. Similarly, the translational part of the study is mostly descriptive and limited by relatively few evaluable biopsies. It should hence be considered hypothesis generating, and especially the presence of a CD30− HRSC-like population requires further study and external validation. Additionally, mechanistic validation of the HDACi-mediated effect on IL21+CD4+ T-cell populations and its potential to unleash a clonally expanded cytotoxic CD8+ T-cell response is pending. The authors are nevertheless to be congratulated for generating and leveraging a high-resolution single-cell atlas of longitudinal HL biopsies, which constitutes an important resource to the field. Once again, a key challenge in the treatment of HL appears to be surmountable by rewiring of the intricate interactions in the HL TME.
Conflict-of-interest disclosure: P.J.B. is an advisor or consultant for Merck Sharp & Dohme, Need Inc, Stemline, and Takeda; holds stock options in Need Inc; has received honoraria from BeiGene, Bristol Myers Squibb (BMS)/Celgene, Merck Sharp & Dohme, Need Inc, Stemline, and Takeda; has received research funding from BeiGene (to the institution [Inst]), BMS (Inst), Merck Sharp & Dohme (Inst), and Takeda (Inst); and reports an Excellence Stipend of the Else-Kröner-Fresenius Foundation.