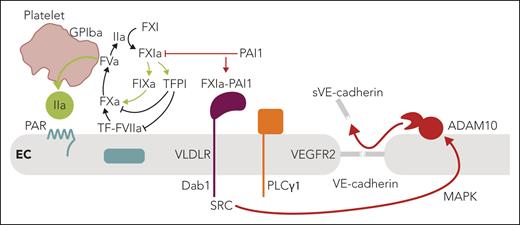
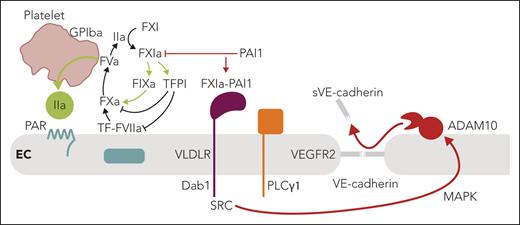
In this issue of Blood, Puy et al1 identify an unexpected endothelial cell barrier disruptive pathway induced by complex formation of factor XIa (FXIa) with plasminogen activator inhibitor 1 (PAI1) and by engagement of very-low-density lipoprotein receptor (VLDLR).
Coagulation FXIa has emerged as a promising therapeutic target to prevent venous and arterial thrombosis. This is supported by epidemiological data showing that FXI deficiency is associated with protection from thromboembolism and from cardiovascular events in the absence of major bleeding complications, in particular intracranial hemorrhage.2 FXI acts as an amplifier of coagulation and is positioned in a feedback loop of FXI zymogen that is proteolytically activated by locally generated thrombin, such as in the tissue factor (TF)-initiated extrinsic coagulation pathway. FXI is also activated directly by FXIIa in the contact pathway and contributes to activation of the plasma kallikrein-kinin system that regulates vascular permeability and inflammation.3 Earlier data from this group4 have shown that targeting FXI with an antibody or antisense oligonucleotide knockdown of FXI synthesis in the liver attenuates atherosclerotic lesion development and protects endothelial barrier function, which is supported by the homotypic interactions of the vascular endothelial cadherin (VE-cadherin). Because these clinically relevant inhibitory strategies may also reduce the availability of FXI for interactions with the contact pathway–activated kallikrein-kinin system, the prior observations in vivo may be explained by interference with these proinflammatory roles of FXIa.
The meticulous current experiments in cultured endothelial cells have revealed a convincing alternative pathway for barrier disruption that does not involve interactions of FXI in the contact pathway. Instead, the FXIa-PAI1 complex engages VLDLR, originally identified as a receptor for reelin in neurodevelopment (see figure). Binding of the protease-serpin complex recruits and phosphorylates the adaptor protein disabled-1 (Dab1) to subsequently---through src kinase---activate phospholipase Cγ1 (PLCγ1) and cross-activate the vascular endothelial cell growth factor receptor 2 (VEGFR2). The resulting activation of the MAP kinase pathway then leads to a disintegrin and metalloproteinase 10 (ADAM10)-mediated shedding of the VE-cadherin extracellular domain and disruption of endothelial barrier function. In an established primate model of sepsis, Puy et al also show that antibody inhibition of FXI activation decreases circulating levels of shed VE-cadherin associated with improved barrier function. Although these data are intriguing, there are alternative pathways by which attenuation of coagulation activation by targeting FXIa may confer endothelial protection in vivo.
Summary of the key findings by Puy et al and the connections to vascular thromboinflammation. FXIa is at the center of coagulation amplification and thromboinflammatory circuits, leading to vascular endothelial dysfunction. In the context of local coagulation activation by TF leading to thrombin (IIa) generation, FXI is activated on the platelet surface dependent on GPIba. In addition to the resulting amplification of thrombin generation through intrinsic FIX activation, FXIa also inactivates TFPI to amplify coagulation locally. PAI1 is a major inhibitor of FXIa on the endothelial cell surface and dampens coagulation. In contrast, engagement of VLDLR by the FXIa-PAI1 complex triggers a barrier-disruptive pathway through intracellular recruitment of Dab1, src kinase (SRC), and PLCγ1-dependent MAP kinase activation, leading to ADAM10-mediated proteolytic shedding of VE-cadherin and barrier disruption. Therapeutic targeting of FXIa may therefore have vascular protective effects in addition to dampening coagulation and thrombin-mediated barrier-disruptive PAR signaling. ADAM10, disintegrin and metalloproteinase 10; EC, endothelial cell; F, factor; GPIba, thrombin receptor glycoprotein Ibα; PAR, protease-activated receptor; PLCγ1; phospholipase Cγ1; TF, tissue factor; TFPI, TF pathway inhibitor; PAI1, plasminogen activator inhibitor 1; VE-cadherin, vascular endothelial cadherin; VLDLR, very-low-density lipoprotein receptor. Professional illustration by Somersault18:24.
Summary of the key findings by Puy et al and the connections to vascular thromboinflammation. FXIa is at the center of coagulation amplification and thromboinflammatory circuits, leading to vascular endothelial dysfunction. In the context of local coagulation activation by TF leading to thrombin (IIa) generation, FXI is activated on the platelet surface dependent on GPIba. In addition to the resulting amplification of thrombin generation through intrinsic FIX activation, FXIa also inactivates TFPI to amplify coagulation locally. PAI1 is a major inhibitor of FXIa on the endothelial cell surface and dampens coagulation. In contrast, engagement of VLDLR by the FXIa-PAI1 complex triggers a barrier-disruptive pathway through intracellular recruitment of Dab1, src kinase (SRC), and PLCγ1-dependent MAP kinase activation, leading to ADAM10-mediated proteolytic shedding of VE-cadherin and barrier disruption. Therapeutic targeting of FXIa may therefore have vascular protective effects in addition to dampening coagulation and thrombin-mediated barrier-disruptive PAR signaling. ADAM10, disintegrin and metalloproteinase 10; EC, endothelial cell; F, factor; GPIba, thrombin receptor glycoprotein Ibα; PAR, protease-activated receptor; PLCγ1; phospholipase Cγ1; TF, tissue factor; TFPI, TF pathway inhibitor; PAI1, plasminogen activator inhibitor 1; VE-cadherin, vascular endothelial cadherin; VLDLR, very-low-density lipoprotein receptor. Professional illustration by Somersault18:24.
In addition to amplifying intrinsic coagulation by activating primarily FIX, but less efficiently also FV and FX, FXIa has other proteolytic targets that typically dampen thrombin generation and result in barrier disruption on endothelial cells.5 These include TF pathway inhibitor (TFPI) that acts as an inhibitor not only of coagulation, but also TF-dependent endothelial protease-activated receptor (PAR) signaling.6 TFPI has also been shown to interact with endothelial cell–expressed VLDLR suppressing proliferation and angiogenesis7 and to modulate VEGFR2 signaling.8 These data suggest that extrinsic and intrinsic coagulation pathways regulate endothelial cell function through multiple interactions with VLDLR.
Coagulation activation on endothelial cells also triggers cytoprotective signaling after thrombin sequestration by thrombomodulin and endothelial cell protein C receptor (EPCR) and PAR1-dependent signaling of the anticoagulant protein C. Remarkably, the activated protein C/EPCR/PAR1 signaling complex counteracts thrombin-induced endothelial barrier disruption by recruiting apolipoprotein E receptor 2 (ApoER2, LRP8), an alternative receptor for reelin in neurodevelopment. LRP8 engagement causes Dab1 phosphorylation and phosphatidylinositol-3 kinase–dependent Akt signaling,9 suggesting that the newly identified FXIa-PAI1 barrier disruptive function alters the signaling balance of closely related receptors and thereby suppresses cytoprotective signaling mediated by the anticoagulant pathway.
FXIa may also indirectly cause barrier dysfunction by cleaving alternative substrates---for example, prochemerin, which promotes chemotaxis and vascular infiltration of leukocytes or complement components that amplify thromboinflammation.5 Remarkably, LRP8 has also been implicated as a receptor for FXI on platelets,5 and sequestration of FXI by platelet-expressed LRP8 may regulate the availability of FXI for interaction with endothelial cell–expressed VLDLR. Platelets are crucial sites for coagulation activation in the context of vascular inflammation. Platelets at the endothelial interface support FXIIa-independent FXI activation by thrombin that is recruited to glycoprotein Ibα.10 Although FXIa has been pursued as an excellent target for antithrombotic therapy, the platelet glycoprotein Ibα–dependent localized amplification of coagulation by thrombin-mediated FXI activation fuels vascular inflammation, particularly in the context of neurohormonal activation involving angiotensin II–driven initiation of the TF pathway.10 Targeting FXIa in this context also significantly reduces platelet procoagulant hyperreactivity, attenuates endothelial dysfunction, and prevents inflammatory vascular and renal damage.
Although clinical trial data on thrombosis prevention by FXI depletion or antibody-mediated inhibition of FXIa show promise, the clinical development of small-molecule protease inhibitors targeting the FXIa active site have so far produced inconclusive results. The efficacy of the different inhibitory strategies to affect the interactions of FXI in the kininogen-kinin system and interfere with platelet-localized activation of FXI in TF-initiated coagulation is currently being explored, but it remains unclear. Puy et al raise additional questions on how targeting FXIa and prevention of FXIa-PAI1 complex formation, which should also be achieved by small-molecule antagonists, can alter inflammatory signaling and potentially detrimental effects of excessive FXIa activation in cardiovascular diseases.
Conflict-of-interest disclosure: The authors declare no competing financial interests.