Visual Abstract
With the global growing older adult population, clinicians face the common, yet complex challenge of how to evaluate and manage anemia in this population. Older age predisposes to common causes of anemia such as nutritional deficiencies, inflammatory disorders, chronic kidney disease, and hematologic malignancies. Failure to diagnose and appropriately manage anemia may result in decreased quality of life, impaired cognition, impaired mobility, and increased mortality. Anemia diagnosis in older adults presents a diagnostic conundrum because anemia may have a single cause, may be multifactorial, or may have no apparent cause even after an extensive evaluation. We believe a systematic approach to diagnosis ensures appropriate testing and avoids the pitfall of undertreatment and overtreatment. In this article we present our recommended approach through common scenarios for the management of anemia in the older adult.
Introduction
Anemia is common in older adults and increases with advancing age.1,2 In the United States, up to 17% of individuals aged >65 years experience anemia.3 The World Health Organization (WHO) defines anemia as a hemoglobin level of <12.0 g/dL for women and of <13.0 g/dL for men.4 This WHO definition of anemia is a population-based threshold, which may not fully capture clinically relevant changes in hemoglobin.5 Older adults who do not meet the WHO definition of anemia may still experience declines in hemoglobin that are associated with increased risk of morbidity and mortality.5 In older adults, approximately a third of anemia cases are due to nutritional deficiencies, a third are due to anemia of chronic diseases [anemia of inflammation (ACI) (also called anemia of chronic disease) and/or chronic kidney disease (CKD)], and a third of cases are unexplained anemia of aging (UAA).6,7 As the older adult population continues to grow around the world, a concomitant rise in older-adult anemia suggests clinicians will benefit from a systematic approach to anemia management in older patients.
Causes of anemia in older adults
Definitive determination of a cause of anemia in older adults is difficult; furthermore, anemia may be multifactorial.8 Potential reasons why anemia is more common in older adults compared with younger adults, are because of hallmarks of aging such as hematopoietic stem cell exhaustion, cellular senescence, and dysregulated nutrient sensing.9 Older adults are more likely to develop gastrointestinal disorders leading to blood loss and malabsorption that may be compounded by anticoagulant or antiplatelet agents as well as a decline in nutrient intake.10 Chronic inflammation can be related to established comorbid illnesses (eg, rheumatoid arthritis [RA] and advanced cancer) and to some extent from aging alone. Inflammation leads to dysregulation in iron metabolism and a blunted hematopoietic response to erythropoietin (EPO).11 There is also a decline in nephron size and number that occurs with age that results in reduced EPO production in individuals with CKD. Age-associated decline in testosterone levels can have negative effects on erythropoiesis, thus, increasing the risk of anemia in older men.12 Undiagnosed myelodysplastic syndrome (MDS) and myeloid precursor conditions such as clonal cytopenia of unknown significance (CCUS) are increasingly recognized as potential causes of anemia.13
Does anemia have adverse effects in older adults?
There have been several studies highlighting the clinical relevance of anemia in older adults. Mild anemia has been associated with poor physical performance, lower muscle strength, and depression and is independently associated with increased mortality.8,14 Anemia also increases the risk of falls and hospitalizations and can lead to a decline in activities of daily living and decreased quality of life (QOL).15 Even in high-functioning older adults, anemia is associated with a 4-fold increase in risk for impaired executive function.16 In a large population-based study, anemia was associated with impaired health-related QOL and poor survival in older adults aged ≥60 years but not in younger adults.17 In addition, women with hemoglobin levels between 12 and 13 g/dL (above the WHO definition of anemia) also experienced lower health-related QOL.17 As we have few anemia corrective therapies, evidence that treating mild anemia or UAA attenuates these outcomes is limited.
The objective of this article is to present a case-based approach to management of anemia in the older adult population.
Case 1
An 80-year-old man with a history of hypertension and coronary artery disease (CAD) who had a non-ST-elevation myocardial infarction a year ago, underwent percutaneous coronary intervention, and was on dual-antiplatelet therapy was referred for evaluation of anemia. He has had anemia for >10 years with hemoglobin of ∼12 g/dL. He tolerated exercise by bicycling without any change in endurance over the past several years. He denied history of gastrointestinal bleeding and has had a negative fecal occult blood test (FOBT). No family members had hematologic disorders. He had an esophagogastroduodenoscopy (EGD) and colonoscopy 3 years ago, which showed no active bleeding sites. Colonoscopy only revealed diverticulosis and nonbleeding internal hemorrhoids.
Laboratory tests showed hemoglobin of 12.1 g/dL, mean corpuscular volume (MCV) of 93 fL, platelet count of 206 × 109/L, white blood cell count (WBC) of 7.5 × 109/L, estimated glomerular filtration rate (eGFR) of 55 mL/min per 1.73m2, vitamin B12 at 408 pg/mL (reference 123-730 pg/mL), folate at 39 ng/mL (reference >5.2 ng/mL), iron at 69 μg/dL (reference 45-150 μg/dL), total iron binding capacity (TIBC) of 270 μg/dL (reference 261-478 μg/dL), ferritin at 91 ng/mL (reference 11-204 ng/mL), transferrin saturation (TSAT) of 26% (reference 15% to 55%), and normal thyroid stimulating hormone (TSH) at 3.06 μIU/mL (reference 0.34-5.66 μIU/mL). The peripheral smear showed no red blood cell (RBC) abnormalities or dyspoiesis.
Discussion of case 1
This is a case of an older adult with a long-standing history of normocytic anemia and no overt etiology on routine testing. This is a common consult in hematology clinics and raises the question of both the necessary and sufficient evaluation. A focused history for bleeding or predisposition to bleeding (eg, antiplatelet or anticoagulants); major medical conditions related to inflammation, cancer, or gastrointestinal malabsorption; and personal or family history of anemia are important. The depth and duration of anemia are critical. In older age, a slow decline in hemoglobin of around 1 g/dL over 15 years can be expected.18
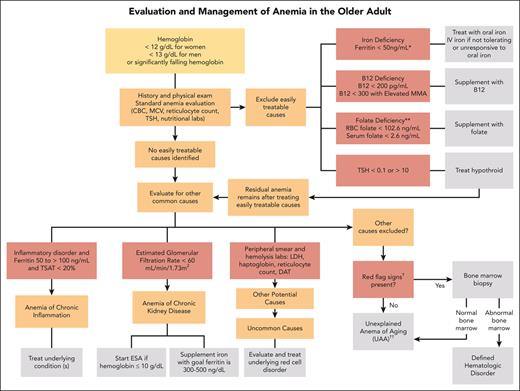
In the absence of findings to direct the evaluation by history, we embark on a standard laboratory evaluation of anemia when testing has not been recently performed. These include a complete blood count (CBC) with differential, reticulocyte count, and peripheral smear review (Figure 1). The peripheral smear specifically assesses for RBC abnormalities, MDS, and leukemia disorders.
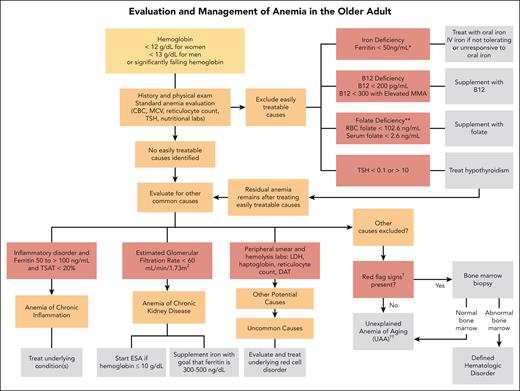
Algorithm for evaluation and treatment of anemia in older adults. The evaluation and management of anemia in the older adult start with a standard anemia evaluation to exclude easily treatable causes such as nutritional deficiencies and thyroid disease. If easily treatable causes are excluded or residual anemia remains after treatment, then continue to evaluate for other causes of anemia. If all other causes of anemia are excluded, then the patient may meet criteria for UAA. ∗Ferritin levels increase with age and patients with conditions such as chronic heart failure or inflammatory disorders may experience absolute iron deficiency at ferritin levels up to 100 μg/L and as high as 300 μg/L if the TSAT is <20%. ∗∗Folate is an uncommon cause of nutritional deficiencies but can be seen in patients with excessive alcohol use and poor nutrient intake. †Red-flag signs include a MCV of >96 fL, an absolute neutrophil count of <1000 cells/μL, platelet count of <120 × 109/L, a family history of blood cancers, abnormal peripheral smear, significant anemia at <9 g/dL, and worsening unexplained anemia. ††Overlap exists between UAA and ICUS. UAA is a diagnosis of exclusion, made only in older adults, whereas ICUS may be diagnosed in patients of any age with ≥1 cytopenia and clonal hematopoiesis with VAF of ≤2%. We favor UAA categorization in cases with mild anemia (often hemoglobin of <2 g/dL below lower limit of normal), no other cytopenia, no other cause, and if blood and/or BM testing is performed, absence of dyspoiesis or hematologic malignancy. DAT, direct antiglobulin test; MMA, methylmalonic acid.
Algorithm for evaluation and treatment of anemia in older adults. The evaluation and management of anemia in the older adult start with a standard anemia evaluation to exclude easily treatable causes such as nutritional deficiencies and thyroid disease. If easily treatable causes are excluded or residual anemia remains after treatment, then continue to evaluate for other causes of anemia. If all other causes of anemia are excluded, then the patient may meet criteria for UAA. ∗Ferritin levels increase with age and patients with conditions such as chronic heart failure or inflammatory disorders may experience absolute iron deficiency at ferritin levels up to 100 μg/L and as high as 300 μg/L if the TSAT is <20%. ∗∗Folate is an uncommon cause of nutritional deficiencies but can be seen in patients with excessive alcohol use and poor nutrient intake. †Red-flag signs include a MCV of >96 fL, an absolute neutrophil count of <1000 cells/μL, platelet count of <120 × 109/L, a family history of blood cancers, abnormal peripheral smear, significant anemia at <9 g/dL, and worsening unexplained anemia. ††Overlap exists between UAA and ICUS. UAA is a diagnosis of exclusion, made only in older adults, whereas ICUS may be diagnosed in patients of any age with ≥1 cytopenia and clonal hematopoiesis with VAF of ≤2%. We favor UAA categorization in cases with mild anemia (often hemoglobin of <2 g/dL below lower limit of normal), no other cytopenia, no other cause, and if blood and/or BM testing is performed, absence of dyspoiesis or hematologic malignancy. DAT, direct antiglobulin test; MMA, methylmalonic acid.
We check for nutritional deficiencies with iron studies and vitamin B12 and folate levels with the following caveats: iron deficiency often reflects bleeding rather than true nutritional deficiency; low B12 does not establish B12-deficient anemia without correction after B12 treatment; and folate deficiency is rare, prompting some clinicians to omit it unless risk factors are identified. In addition, serum folate levels may reflect recent dietary intake of folate, which may be from a variety of sources such as fortified foods or supplements.19 RBC folate is more reflective of long-term folate status; however, methods and reference ranges vary in each laboratory and testing is not widely available in every laboratory.19 Finally, we check thyroid studies and eGFR and perform a C-reactive protein (CRP) test. Such studies facilitate determination of other causes of anemia that may be contributory such as hypothyroidism, renal insufficiency, or anemia of inflammation, respectively.20
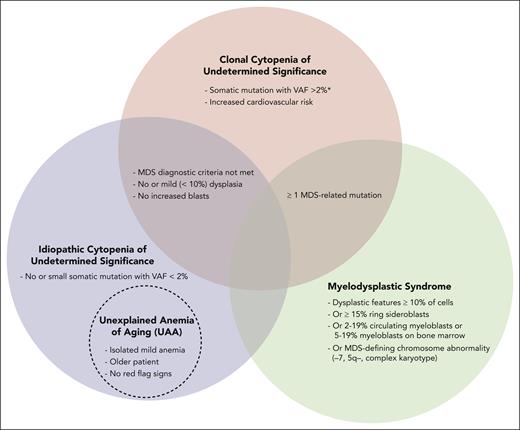
For stable mild anemia, we do not recommend routine bone marrow (BM) biopsy. If there are “red flag signs,” which include MCV >96 fL, additional cytopenias (absolute neutrophil count <1000 cells per μL and/or platelets <120 × 109/L), family history of blood cancers, dysplasia or blasts on peripheral smear or severe (hemoglobin <9 g/dL) or worsening unexplained anemia, we generally perform a BM aspirate and biopsy. Careful morphologic inspection should be supplemented by cytogenetics with or without fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS) mutational studies to differentiate between MDS, CCUS, and idiopathic cytopenia of undermined significance (ICUS).21,22 MDS is diagnosed if MDS diagnostic criteria are met.22 If the patient has anemia, does not meet the MDS diagnostic criteria, and has somatic mutations associated with hematologic malignancies on cytogenetics, FISH, or NGS, then the diagnosis is consistent with either CCUS if variant allele frequency (VAF) is >2% or ICUS if VAF is <2% (Figure 2).7,23 Overlap exists between ICUS and UAA and possibly CCUS, depending on the CCUS definition. We favor UAA if the patient is older, they have an isolated mild anemia (eg, hemoglobin less than 2 g/dL below the lower limit of normal), and, if performed, no suspicious findings on smear or BM studies of a hematologic malignancy. In this case UAA was diagnosed after other causes of anemia were excluded (Table 1).7
Relationship of UAA to ICUS, CCUS, and MDS. ∗MDS-related mutations detected by NGS, chromosome analysis, or FISH.
Relationship of UAA to ICUS, CCUS, and MDS. ∗MDS-related mutations detected by NGS, chromosome analysis, or FISH.
Management of UAA
There is no consensus on how to treat UAA because the pathogenesis remains unclear and is likely heterogenous.7 The data on expected EPO levels in patents with UAA are conflicting.20,24 Patients with UAA generally have inappropriately low levels of endogenous EPO after controlling for hemoglobin, comorbities, and eGFR.24,25 Nevertheless, higher EPO levels may occur because of a blunted BM response to EPO, requiring higher endogenous EPO to increase hemoglobin production because of deficiencies in the hypoxia/erythropoietin sensing mechanism that occur with age.20 Despite these data, the role of erythropoiesis stimulating agents (ESA) remains unclear in UAA. For patients who have concurrent CKD and hemoglobin values below 10 g/dL, ESA treatment may be considered following the Kidney Disease Improving Global Outcomes guidelines, with the exception that one should first ensure that no other treatable or confounding anemia etiology exists.26
Investigators have studied IV iron in UAA.27 In a randomized controlled trial, adults aged ≥65 years with UAA and ferritin ranging from 20 to 200 ng/mL treated with a 5-week course of weekly IV iron sucrose 200 mg had a small but statistically significant hemoglobin increase 0.39 ± 0.46 g/dL at 12 weeks.27
For our patient with a stable chronic anemia and no evidence of serious other medical conditions, we recommend observation with annual CBC with differential or sooner should symptoms emerge. Although the above evaluation may seem significant, the systematic study provides reassurance to avoid unnecessary serial testing and anxiety generated from repeated gastrointestinal endoscopic studies, BM testing, and/or full body imaging for occult malignancies.
Case 1 educational points
UAA is the cause of anemia in about a third of anemia cases in older adults and is a diagnosis of exclusion. The pathogenesis is unclear, possibly driven by multiple age-related changes. The anemia of UAA is frequently mild, and we recommend observation without BM testing.
Case 2
A 68-year-old woman with a history of type 2 diabetes mellitus, hypertension, CAD on aspirin, repaired abdominal aortic aneurysm, hyperlipidemia, and osteoporosis was referred for evaluation of anemia. She has been on a vegan diet for 3 years. Her physician initiated oral iron sulfate (65 mg of elemental iron), 1 tablet daily. She denied cravings for ice. She denied fatigue but had reduced endurance evident by a new requirement to rest after a short walk. She had been transfused only once in her life (during the aneurysm repair 10 years ago). She had no family history of blood cancers.
Laboratory review showed anemia emerged ∼2 years ago, with most recent hemoglobin of 13.5 g/dL, and an MCV of 89 fL during an annual physical examination 3 years ago. Hemoglobin declined gradually. Her laboratory evaluation showed hemoglobin of 11.3 g/dL, MCV of 83 fL, serum iron at 25 μg/dL, TIBC of 396 μg/dL, TSAT of 7%, and serum ferritin at 25 ng/mL. FOBT was positive using 3 cards. She underwent an EGD and a colonoscopy and there was no obvious cause of bleeding found other than mild gastritis. She was negative for Helicobacter pylori.
Discussion of case 2
This is a patient with iron deficiency anemia (IDA) who has been on oral iron for 2 years and has not had a sufficient response. Nutritional deficiencies, such as B12, folate, and iron deficiency, have been implicated in approximately one-third of older adults with anemia, with IDA being the most common.6 Older adults with iron deficiency may report fatigue, restless leg syndrome, and/or pica, which is a craving for nonnutritive substances.28 Although overt and occult gastrointestinal bleeding accounts for the majority of IDA in older adults, numerous causes may contribute including nongastrointestinal blood loss (eg, hematuria), intravascular hemolysis, malabsorption, and poor dietary intake. Older adults are at higher risk of gastrointestinal lesions and colorectal cancer.29,30 In a prospective study in which 100 consecutive patients with IDA underwent colonoscopy and EGD, 62 patients had at least 1 gastrointestinal lesion responsible for blood loss.31 Considerations for ineffective oral iron therapy for IDA include bleeding in excess of iron repletion, nonadherence especially in the setting of intolerance, malabsorption, or insufficient dosing.
To evaluate gastrointestinal bleeding, we usually refer for colonoscopy and/or EGD. We frequently recommend FOBT, recognizing that FOBT alone is insufficient for excluding gastrointestinal bleeding, with a sensitivity of 58% at detecting the source of IDA.32 FOBT is more appropriate for colorectal cancer screening in asymptomatic people without anemia.32 We refer for endoscopic evaluation irrespective of FOBT when IDA is suspected unless another etiology for IDA is established. In frail older adults, the decision to perform an endoscopy is often case-by-case with consideration for the person’s life expectancy.33 It should be a shared decision with the patient and caregiver; a positive FOBT suggests a higher yield from endoscopy.34
Patients with IDA may present with microcytic or normocytic hypochromic RBCs. Nearly 70% of older adults (aged ≥65 years) with IDA present with normal MCV.1 Laboratory evaluation includes obtaining a CBC, reticulocyte count, serum iron level, TIBC (represents the ability to bind iron to transferrin), TSAT (ratio of serum iron to TIBC), and ferritin (reflects iron stores). Individuals with iron deficiency typically have low serum iron, low TSAT, low ferritin, and high TIBC. Timing and fasted state should be considered when iron studies are collected.35 A TSAT of <20% suggests that the iron supply is not adequate for RBC production and a very low TSAT of <15% is consistent with iron deficiency. Compared with lower ferritin cutoffs, a ferritin level of <50 ng/mL is suspicious for iron deficiency in older adults because this is a more reliable predicter of iron deficiency in older populations.30,36 Intermediate ferritin levels (the range from 30 to 100 ng/mL) pose difficulties, particularly in older adults with chronic diseases. Ferritin levels increase with age and patients with conditions such as chronic heart failure or inflammatory disorders may experience absolute iron deficiency even at ferritin levels of up to 100 μg/L and as high as 300 μg/L if the TSAT is <20%.37-39
Patients may also present with deficiencies in B12, folate, and other nutrients such as copper that may cause anemia, macrocytosis, dysplastic features on peripheral smear, and neurologic symptoms.8 In countries with folate supplementation, deficiencies are rare but should be suspected in those with severe malnourishment including severe alcohol overuse. Adults aged >60 years are at highest risk for developing pernicious anemia (autoantibodies against intrinsic factor and parietal cells).40 A B12 level of <300 pg/mL suggests B12 deficiency but does not necessarily indicate B12-deficient anemia. Elevated levels of serum methylmalonic acid suggest B12 deficiency and warrants a diagnostic trial of B12.15 Methylmalonic acid assays are not readily available in all laboratories and correction of borderline B12 deficiency does not always lead to hemoglobin improvement. In cases of borderline B12 deficiency (levels 200-300 pg/mL), we recommend a therapeutic trial of B12, considering the low cost and potential benefit of supplementation. Symptoms of B12 deficiency can be reversed with appropriate supplementation. Severe pernicious anemia should be corrected slowly to reduce the risk of sudden death related to shifts in potassium from extracellular fluid into maturing RBCs.15 Older adults with mild B12 deficiency with normal absorption usually respond to an oral B12 dose of 1000 μg, daily.41 Patients with impaired absorption often require parenteral B12 indefinitely.42,43
Management of IDA
Management of IDA in older adults includes iron supplementation and addressing the cause of IDA. Repletion of iron using oral iron formulations, typically containing 50 to 65 mg of elemental iron, can be challenging for many older adults because of side effects such as constipation, diarrhea, and abdominal cramping.44
A misconception exists that high-dose oral iron, 3 times a day, represents the best way to treat IDA. The absorption of oral iron is optimal when it is taken on an empty stomach (1 hour before a meal or 2 hours after a meal). Alternate day dosing of oral iron also allows better absorption because there is an acute rise in hepcidin for 24 hours after oral iron is ingested.45 For management of IDA in older adults, 1 study found equivalent hemoglobin response and iron repletion, but with less toxicity, with once daily iron at 15 mg compared with 50 mg or 150 mg of iron daily.46 Consequently, in patients who do not tolerate formulations with >50 mg of elemental iron, a switch to low-dose iron formulations should be considered.46
IV iron is recommended for patients who have an intolerance or inadequate response to oral iron or malabsorption conditions such as history of gastric bypass. Numerous IV iron formulations exist. Iron replacement has been previously reviewed.47 From a practical perspective, institutions may have preferred IV iron formulations.
The patient in case 2 was treated with 1 dose of IV low–molecular weight dextran 1000 mg. Her walking endurance improved, and she achieved a normal hemoglobin level. She continued oral ferrous sulfate every other day because of an ongoing need for aspirin for secondary prevention of CAD. We concluded that the patient had occult gastrointestinal bleeding from either gastritis or an occult source. Small-bowel interrogation is a consideration now or in the future. We recommended at least annual monitoring for development of new symptoms or recurrence of IDA.
Case 2 educational points
In older adults with IDA, it is important to determine the cause of iron deficiency and review how the patient is taking oral iron and adherence to iron supplementation and side effects. Counsel patients on methods of taking iron that will promote optimal absorption, such as alternate day dosing and taking it on an empty stomach. IV iron is generally safe for older adults and should be used in patients who are unable to tolerate, or respond inadequately to, oral iron supplementation.28
Case 3
A 75-year-old woman with stage III CKD, a gastric bypass 20 years ago, hyperlipidemia, and RA on daily low dose prednisone was referred for evaluation of anemia. Her weight in clinic was 102 lb (46 kg). She has been on a compounded multivitamin and B12 injections from her bariatric team. She reported severe fatigue and feeling winded with prolonged walking. She denied hematochezia or melena.
Her laboratory evaluation showed a WBC of 8.7 × 109/L, hemoglobin of 8.4 g/dL, MCV of 101 fL, platelets at 204 × 109/L, reticulocyte count of 53.9 × 109/L, creatinine at 2.4 mg/dL, eGFR at 20 mL/min per 1.73m2, B12 of >1500 pg/mL, ferritin at 50 ng/mL, iron at 76 μg/dL, TIBC of 383 μg/dL, TSAT of 20%, folate at 9.4 ng/mL, TSH at 2.44 μIU/mL, and copper at 134 μg/dL.
Her peripheral smear showed macrocytosis.
After the initial workup, additional laboratory testing revealed CRP of 22.5 mg/dL (reference ≤0.6 mg/dL), EPO of 24 mIU/mL (reference 12-13 mIU/mL), no M-spike on serum protein electrophoresis, FOBT negative, and lactate dehydrogenase (LDH) of 202 U/L (reference 100-200 U/L).
Her colonoscopy was normal except for nonbleeding internal hemorrhoids. Her EGD was normal with no H pylori.
Analysis of BM biopsy and aspirate, myeloid NGS, FISH, and chromosome showed a normocellular marrow at 20% to 30%, no dyspoiesis, and a normal karyotype.
Discussion of case 3
This is a case of multifactorial anemia, which is a common finding in older adults. Iron-restricted erythropoiesis from malabsorption, anemia of CKD, and anemia of chronic inflammation (ACI) from RA and CKD. Patients with multifactorial anemia should be evaluated and managed in a stepwise approach. Our patient presented with macrocytosis, which can occur in the setting of megaloblastic anemia, MDS, hypothyroidism, alcoholism, and reticulocytosis. B12 and folate deficiencies were excluded, her thyroid studies were normal, and her BM biopsy was not consistent with a primary hematologic neoplasm.
The markedly elevated CRP suggests an active inflammatory process, probably primarily from RA and CKD. ACI is a common cause of anemia in older adults.15 Iron-restricted erythropoiesis constitutes a major component of ACI. Increased hepcidin (a hepatically synthesized acute phase reactant) induces degradation of ferroportin in enterocytes, macrophages, and hepatocytes.48 The reduction in ferroportin reduces intestinal iron absorption and decreases release of iron from macrophages into circulation, which leads to lower serum iron levels and iron-restricted erythropoiesis, even when the body’s iron stores are adequate.48
The patient also has concurrent anemia of CKD. There is a decline in hemoglobin as eGFR declines, which is caused by a reduction in endogenous EPO, decreased hematopoietic response to EPO because of uremic toxins, and absolute and functional iron defiicicency.49 Severe EPO deficiency occurs when eGFR is <30 mL/min per 1.73m2.
In older adults with inflammation and CKD, ferritin levels indicating iron-restricted anemia can be variable; ferritin <100 μg/dL if not 200 μg/dL may be amenable to IV iron.6
Management of multifactorial anemia
After ascertaining potential causes, we first aim to treat with the most specific agent. For example, potential IDA will be treated first. Uncontrolled comorbid inflammatory illness should be appropriately managed. Anemia can best be ascribed to remaining causes, such as CKD after sequential exclusion or optimal management of other conditions. For residual anemia with CKD, we adhere to the Kidney Disease Improving Global Outcomes guidelines on hemoglobin threshold and targets for ESA.50 Importantly, we do not aim for normalization of hemoglobin levels when using ESAs and only initiate at hemoglobin level of <10 g/dL and avoid exceeding hemoglobin levels of >11 g/dL to minimize cardiovascular complications.51
Because the patient has a history of bariatric surgery, ACI, and anemia of CKD, we continued nutritional optimization with B12 injections, started IV iron to maintain ferritin between 300 and 500 ng/mL, and deferred ESA because of stable hemoglobin at 9.5 g/dL. Symptoms of fatigue and exercise tolerance substantially improved.
Case 3 educational points
Anemia in older adults is often multifactorial and should be evaluated and managed with a sequential approach. Nutritional deficiencies should also be corrected and maintained with special attention to maintaining adequate iron stores in patients treated with ESAs. ACI is managed by treating the underlying chronic inflammatory conditions whenever possible.
Case 4
A 60-year-old woman with a history of CKD, hypertension, and fibromyalgia was referred for anemia and left upper quadrant (LUQ) abdominal pain that started during a trip to Denver, Colorado, a month ago. She recalled having intermittent pain in her legs and back every few years since childhood. As no cause had been found, fibromyalgia had been diagnosed. A few days after her trip, the LUQ pain started to improve but she had ongoing fatigue. For anemia, she had been prescribed oral iron therapy intermittently for the past 30 years. She had no family history of blood disorders.
Her hemoglobin was 8.2 g/dL; hematocrit, 24%; MCV, 75 fL; platelets, 132 × 109/L; WBC, 8.6 × 109/L; and reticulocyte count, 94.0 × 109/L.
Peripheral smear showed target cells and dense rectangular crystals.
Computed tomography of the abdomen and pelvis showed progressive splenic atrophy with areas of heterogeneous low attenuation, likely representing evolving infarcts.
Given splenic infarcts, hemoglobin electrophoresis was performed showing S, 54%; C, 44%; and A2, 2% consistent with a diagnosis of hemoglobin sickle cell disease (SCD).
Screening for SCD complications revealed a urine microalbumin-to-creatinine ratio of 339.8 mg/g (reference, <30 mg albumin/1 g creatinine), eGFR of 29 mL/min per 1.73 m2, and creatinine of 1.9 mg/dL (reference, 0.4-1.0 mg/dL), concerning for sickle cell nephropathy.
Her iron was 39 μg/dL; ferritin, 268 ng/mL; TIBC, 218 μg/dL; TSAT, 18%; and LDH, 402 U/L.
Discussion of case 4
When older adults are evaluated for anemia, certain features such as systemic symptoms and severe anemia may suggest more significant underlying causes such as hereditary red cell disorders.25 In a prospective study evaluating causes of unexplained anemia in a cohort of predominantly (69%) African American older adults aged ≥65 years, 4.6% had thalassemia trait, and 2.2% had a hemolytic condition. In clinical practice, patients can present at any age with uncommon causes of anemia such as undiagnosed SCD, thalassemia, hereditary spherocytosis, glucose-6-phosphate dehydrogenase deficiency, and autoimmune hemolytic anemia.6,15 A thorough medical and family history, review of symptoms, and peripheral blood smear will help determine whether additional workup for inherited or acquired red cell disorders is indicated.
Membranopathies such as hereditary spherocytosis may go undiagnosed until older age if they cause infrequent or no major hemolytic episodes.52
In patients with MCV of <80 fL and no iron deficiency, a diagnosis of thalassemia is highly likely. β-globin disorders can be confirmed with hemoglobin electrophoresis or high-performance liquid chromatography. The presence and type of α-globin mutation can be confirmed with α-globin gene sequencing. Typically, no treatment is required for thalassemia trait.
SCD is the most common severe inherited blood disorder in the United States. SCD is caused by a point mutation in the β-globin gene that leads to vaso-occlusion, thus causing complications such as strokes, renal disease, cardiopulmonary complications, and accelerated functional decline.53-55 There are several variants of SCD with hemoglobin SS being the most common. Individuals with hemoglobin-SC or sickle-β+–thalassemia variants are more likely to go undiagnosed for decades because they have fewer complications early in life, have a relatively higher hemoglobin level (usually 10-12 g/dL), and have a longer life expectancy compared with individuals with hemoglobin SS.56,57
Older adults with SCD may develop progressive anemia due to both SCD and age-associated changes such as CKD, stem cell exhaustion due to a lifetime of hemolysis, and increased RBC turnover.58,59 Hematologic malignancies are also more common in individuals with SCD compared with the general population, and clonal hematopoiesis may also be more common in adults with SCD, occurring at an earlier age.60-62
Management of uncommon causes of anemia
Management of anemia in older adults with SCD and other inherited red cell disorders includes management of acute and chronic causes. Fortunately, our patient’s splenic infarction and LUQ pain resolved. Her hemoglobin returned to her baseline at 9.8 g/dL and LDH level normalized, which were also associated with resolution of her fatigue. She did not require RBC transfusion. We discontinued iron therapy given the improvement in hemoglobin and TSAT. After acute complications are controlled, routine SCD management strategies should be performed. Hydroxyurea has been shown to increase hemoglobin, reduce vaso-occlusive events, and improve survival in individuals with SCD.63,64 Voxelotor may also improve chronic anemia in individuals with SCD.65
Case 4 educational points
Some older adults with anemia may have uncommon causes, such as undiagnosed hemoglobinopathies or hemolytic anemias. A systematic approach with review of medical history, family history, and laboratory perturbations, inclusive of the peripheral smear, should provide clues to diagnose rarer causes of anemia in older adults. The anemia may be compounded by typical causes of anemia in older adults (eg, nutritional deficiencies, ACI, CKD, and hematologic malignancies); however, these issues may occur earlier in life.
Conclusion
Anemia is a common sign with protean causes and manifestations. A systematic approach to evaluation and management of anemia ensures appropriate care that may optimize hemoglobin and potentially ameliorate anemia-related morbidity while minimizing unnecessary testing. Nevertheless, further research is necessary because often anemia in older age has no discernible cause or proven therapy.
Acknowledgments
The authors acknowledge the contribution from patients described in these cases and the investigators who led the studies that improve how we care for anemia in older adults.
This work was supported by research funding from the Duke Center for Research to Advance Healthcare Equity (REACH Equity), which is supported by the National Institutes of Health (NIH), National Institute on Minority Health and Health Disparities under award number U54MD012530 and the NIH National Institute on Aging Grants for Early Medical/Surgical Specialists' Transition to Aging Research (GEMSSTAR) award number 1R03AG074054-01 to C.I.O.
Authorship
Contribution: C.I.O., A.S.A., and H.J.C. contributed to the original design, draft, and revisions of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charity I. Oyedeji, Division of Hematology, Department of Medicine, Duke University School of Medicine, 315 Trent Dr, Suite 266, DUMC Box 3939, Durham, NC 27710; email: charity.oyedeji@duke.edu.