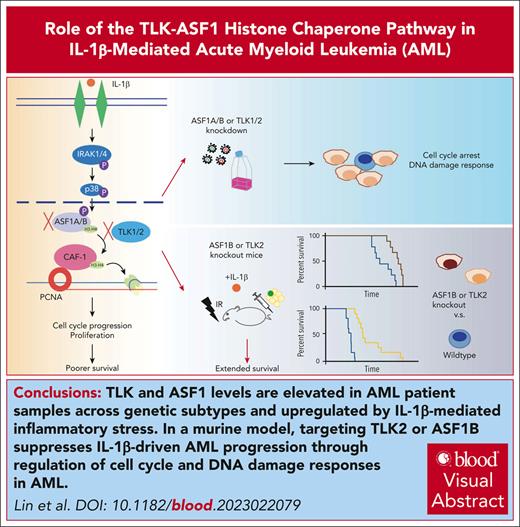
Key Points
TLK and ASF1 levels are elevated in patients with AML across genetic subtypes and upregulated by IL-1β–mediated inflammatory stress.
Targeting TLK2 or ASF1B suppresses IL-1β–driven AML progression through regulation of cell cycle and DNA damage responses in AML.
Visual Abstract
Identifying and targeting microenvironment-driven pathways that are active across acute myeloid leukemia (AML) genetic subtypes should allow the development of more broadly effective therapies. The proinflammatory cytokine interleukin-1β (IL-1β) is abundant in the AML microenvironment and promotes leukemic growth. Through RNA-sequencing analysis, we identify that IL-1β–upregulated ASF1B (antisilencing function-1B), a histone chaperone, in AML progenitors compared with healthy progenitors. ASF1B, along with its paralogous protein ASF1A, recruits H3-H4 histones onto the replication fork during S-phase, a process regulated by Tousled-like kinase 1 and 2 (TLKs). Although ASF1s and TLKs are known to be overexpressed in multiple solid tumors and associated with poor prognosis, their functional roles in hematopoiesis and inflammation-driven leukemia remain unexplored. In this study, we identify that ASF1s and TLKs are overexpressed in multiple genetic subtypes of AML. We demonstrate that depletion of ASF1s significantly reduces leukemic cell growth in both in vitro and in vivo models using human cells. Using a murine model, we show that overexpression of ASF1B accelerates leukemia progression. Moreover, Asf1b or Tlk2 deletion delayed leukemia progression, whereas these proteins are dispensable for normal hematopoiesis. Through proteomics and phosphoproteomics analyses, we uncover that the TLK-ASF1 pathway promotes leukemogenesis by affecting the cell cycle and DNA damage pathways. Collectively, our findings identify the TLK1-ASF1 pathway as a novel mediator of inflammatory signaling and a promising therapeutic target for AML treatment across diverse genetic subtypes. Selective inhibition of this pathway offers potential opportunities to intervene effectively, address intratumoral heterogeneity, and ultimately improve clinical outcomes in AML.
Introduction
Acute myeloid leukemia (AML) is one of the deadliest hematologic malignancies. Genetic heterogeneity within and between patients is an ongoing challenge for treating AML. For decades, conventional chemotherapies have served as the standard of care for patients with AML.1 Despite the development of targeted therapies against common mutations, such as FMS-related tyrosine kinase 3 (FLT3), the emergence of drug-resistant mutations and therapeutic resistance remains a major obstacle.2 Furthermore, the bone marrow microenvironment plays a critical role in AML progression, providing a supportive niche for leukemic cells and contributing to therapeutic resistance.3 Therefore, targeting common pathways regulated by the bone marrow microenvironment may provide a promising therapeutic strategy to overcome intratumoral heterogeneity and therapeutic resistance, and improve the outcomes in patients with AML.
Recent studies have highlighted the association between infectious or autoimmune diseases and an increased risk for myelodysplastic syndrome and AML, underscoring the involvement of the microenvironment in disease development.4-6 Importantly, we, and others, found that the bone marrow microenvironment in AML is inflammation rich and exerts inflammatory stress on healthy and leukemic hematopoietic stem and progenitor cells. Specifically, we have identified that the proinflammatory cytokine interleukin-1β (IL-1β) is elevated in patients with AML, which promotes leukemic cell growth while suppressing the growth of healthy progenitors.7 Furthermore, several downstream effectors of IL-1β signaling, including IL-1RAP, IRAK1, and TRAF6, have been implicated in AML and myelodysplastic syndrome, and targeting these molecules has shown potential in impairing leukemic cell survival.8-10
ASF1A and ASF1B are paralogous histone chaperones responsible for delivering histone H3-H4 to the replication fork during DNA replication, transcription, and DNA damage repair.11,12 These histone chaperones are substrates for Tousled-like kinases 1 and 2 (TLK1 and TLK2), which are paralogous kinases essential for DNA replication and repair.13,14 Phosphorylation of ASF1 by TLKs promotes binding and delivery of the histone H3-H4 dimer.14-17 Notably, elevated expression of both ASF1 and TLK has been observed in a variety of cancers, including breast cancer, and is associated with a poor prognosis.18-20 In this study, we sought to investigate the involvement of the TLK-ASF1 pathway in AML progression. We demonstrated that both TLKs and ASF1s are highly expressed in AML cells compared with healthy cells, and this upregulation was further enhanced under IL-1β–mediated inflammatory stress. Subsequently, we examined the impact of TLK and ASF1 depletion on AML cell growth using in vitro and in vivo models, aiming to evaluate their potential as therapeutic targets in AML. By unraveling the intricate interplay between inflammation and the TLK-ASF1 pathway in AML, our findings contribute to a deeper understanding of AML pathobiology and establish a solid foundation for the development of targeted therapeutic interventions against this devastating disease.
Methods
Primary samples
Peripheral blood or bone marrow of primary AML samples were obtained from Oregon Health and Science University (OHSU) Beat AML biorepositories and patients consented following the institutional review board guidelines. All samples represented unique patients with AML and the clinical, genetic, and demographic information of patient samples are summarized in supplemental Table 1, available on the Blood website. The details for primary sample purification and culturing are described in the supplemental Methods.
Gene expression analyses
Messenger RNA (mRNA) of purified CD34+ cells from patients with AML and healthy donors (purchased commercially from Lonza Inc) was extracted using miRNeasy micro kit following the manufacturer’s protocol. RNA sequencing (RNA-seq) analyses and TaqMan low-density array (TLDA) were used as described in the supplemental Methods.
Cell line generation, cell-based assays, and xenograft study
MOLM-14 cells expressing short hairpin RNA (shRNA) targeting TLK1, TLK2, ASF1A, and ASF1B, and 2 nonspecific controls (NT16 and NT17) were generated using lentivirus. Upon ASF1 and TLK knockdown, colorimetric MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] cell viability, cell growth, cell cycle, and colony formation assays were performed. In vivo, the effect of ASF1 knockdown was assessed using nonobese diabetic/severe combined immunodeficiency IL2Rγ−/− (NSG) mice, and is described in the supplemental Methods.
Mice
All animal studies were approved by OHSU institutional animal care and use committee. Animals were housed at the OHSU semiconventional facility. Asf1bem1(IMPC)J (MMRRC stock 42325), Vav-iCre (JAX stock 008619), and C57BL/6 mice were purchased from The Jackson Laboratory. Tlk2fl/fl mice21 were crossed with Vav-iCre mice to generate Vav-Cre+Tlk2fl/+ and Vav-Cre+Tlk2fl/fl hematopoietic-specific conditional knockout mice. For genotyping, tail tips were collected at weaning and analyzed by quantitative real-time polymerase reaction (Transnetyx).
Bone marrow transplantation
Bone marrow cells were isolated from bones of 6- to 8-week-old Asf1b+/+, Asf1b+/−, Asf1b−/−, Vav-Cre−Tlk2fl/fl, Vav-Cre+Tlk2fl/+, and Vav-Cre+Tlk2fl/fl donor mice. Bone marrow transplantations were performed as described previously22-24 and in the supplemental Methods.
Proteomics and phosphoproteomics
Global proteomics and phosphoproteomics analysis of AML cell line MOLM-14 were performed upon knockdown of ASF1s or TLKs as described in the supplemental Methods.
Statistical analysis
Variables were compared by pairwise Student 2-tailed t test assuming homoscedastic variances. Fisher exact test was used for categorical variables, and Mann-Whitney U test was used for continuous variables. The level of significance was set at P value ≤ .05.
Results
Gene expression profiling identifies ASF1B upregulation in AML progenitors upon IL-1β stimulation
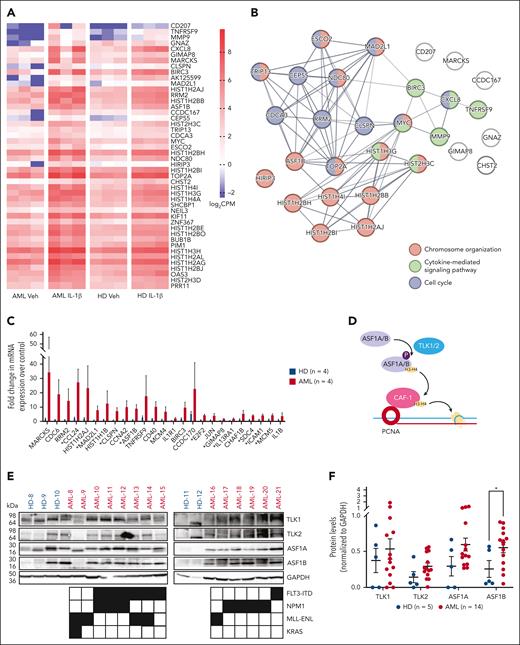
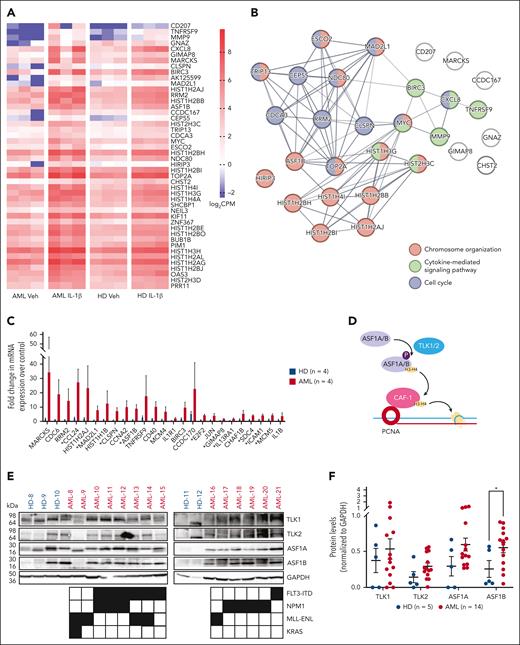
We previously found that aberrant IL-1β signaling promotes the growth of AML progenitors and leukemogenesis.7 To reveal the cell-intrinsic mechanisms downstream of IL-1β signaling in AML, we performed RNA-seq analysis of AML and healthy bone marrow CD34+ progenitors treated with and without IL-1β from 3 independent patient samples. We identified 66 differentially expressed genes in AML progenitors and healthy progenitors upon IL-1β exposure compared with vehicle-treated controls (false discovery rate of <0.05, supplemental Tables 2 and 3). Upon IL-1β stimulation, 46 genes were only upregulated in AML progenitors (Figure 1A), 7 genes were only upregulated in healthy progenitors, and 12 genes were upregulated in both. In addition, VCAN was the only gene that was significantly downregulated in healthy progenitors.
ASF1B is upregulated in AML progenitors upon IL-1β stimulation. (A) RNA-seq analysis was performed using purified CD34+ cells derived from the bone marrow of patients with AML (n = 3) and healthy donors (n = 3). Cells were cultured with 10 ng/mL IL-1β or vehicle control for 2 days. The heat map indicates log2 counts per million (CPM) of differentially expressed genes in vehicle- or IL-1β–treated healthy and AML CD34+ bone marrow cells. (B) STRING-DB network analysis of genes upregulated in IL-1β–treated AML cells vs IL-1β–treated healthy cells. (C) TLDA analysis was performed on AML (n = 4) and healthy CD34+ (n = 4) bone marrow cells treated with or without 10 ng/mL IL-1β for 2 days. The expression of listed genes is shown as a fold difference upon IL-1β stimulation compared with respective vehicle controls in AML and healthy CD34+ bone marrow cells. (D) Schematic representation of the TLK-ASF1 pathway. (E) Immunoblot of TLKs and ASF1s protein expression in CD34+ bone marrow cells from healthy donors (n = 5) and patient-derived AML samples (n = 14) with indicated mutations. (F) Densitometry analysis of the immunoblot is shown and expressed as means ± standard error of the mean (SEM). Student t test was used for statistical analysis. ∗P < .05.
ASF1B is upregulated in AML progenitors upon IL-1β stimulation. (A) RNA-seq analysis was performed using purified CD34+ cells derived from the bone marrow of patients with AML (n = 3) and healthy donors (n = 3). Cells were cultured with 10 ng/mL IL-1β or vehicle control for 2 days. The heat map indicates log2 counts per million (CPM) of differentially expressed genes in vehicle- or IL-1β–treated healthy and AML CD34+ bone marrow cells. (B) STRING-DB network analysis of genes upregulated in IL-1β–treated AML cells vs IL-1β–treated healthy cells. (C) TLDA analysis was performed on AML (n = 4) and healthy CD34+ (n = 4) bone marrow cells treated with or without 10 ng/mL IL-1β for 2 days. The expression of listed genes is shown as a fold difference upon IL-1β stimulation compared with respective vehicle controls in AML and healthy CD34+ bone marrow cells. (D) Schematic representation of the TLK-ASF1 pathway. (E) Immunoblot of TLKs and ASF1s protein expression in CD34+ bone marrow cells from healthy donors (n = 5) and patient-derived AML samples (n = 14) with indicated mutations. (F) Densitometry analysis of the immunoblot is shown and expressed as means ± standard error of the mean (SEM). Student t test was used for statistical analysis. ∗P < .05.
Gene ontology analysis using STRING-DB25 revealed that 46 of 65 upregulated genes in AML progenitors upon IL-1β stimulation belong to biological processes including chromosome organization (ASF1B, MAD2L1, and HIRIP3), cytokine-mediated signaling (TNFRSF9, BIRC3, and MMP9), as well as cell cycle (CDCA3, CEP55, and RRM2; Figure 1B; supplemental Tables 2 and 3). We confirmed our findings from RNA-seq analysis using the TLDA to assess the expression of top differentially expressed genes and genes associated with the IL-1β signaling in additional samples from patients with AML harboring diverse genetic mutations such as FLT3-ITD and NPM1 (supplemental Table 4; Figure 1C). Consistent with our RNA-seq data, TLDA revealed that ASF1B has larger fold changes in AML vs healthy progenitors (Figure 1C). Higher ASF1B expression is associated with poor prognosis in solid tumors, such as breast and cervical cancer.18,26,27 However, its role in hematopoiesis and leukemia is unclear. Therefore, we focused on understanding the role of ASF1B in AML progression at a steady state and under inflammatory stress.
Expression of ASF1 and TLK is upregulated in AML across genetic subtypes
ASF1B and its paralogue ASF1A are regulated by TLK1 and TLK2 (Figure 1D),14 which are amplified in several types of solid tumors.19,20 Therefore, we first quantified the TLK and ASF1 levels in healthy CD34+ progenitor cells, as compared with AML cells derived from patients with common genetic mutations and translocations including FLT3-ITD, NPM1, and MLL-ENL (Figure 1E). We found that ASF1B levels were significantly higher (2.2-fold) in AML than in healthy progenitors. Additionally, the expression of TLK2, TLK1, and ASF1A exhibited an increasing trend (2.1-, 1.4-, and 2-fold, respectively) due to variations in different patient samples (Figure 1E-F). TLK1, TLK2, ASF1A, and ASF1B were also highly expressed across various AML cell lines (supplemental Figure 1A). Accordingly, the DepMap CRISPR database analysis28 shows that most AML cell lines are highly dependent on ASF1B, ASF1A, and TLK2, whereas less dependent on TLK1 (supplemental Figure 1B). Importantly, patients with AML with higher ASF1B expression showed poor survival in the BeatAML database (supplemental Figure 1C). Furthermore, similar to our previous study7 in which IL1R1 expression is not correlative of genetic subtypes, most AML genetic subtypes did not correlate with high ASF1B expression in AML29 except the presence of KRAS mutations in patients was correlated with higher ASF1B expression (supplemental Figure 1D). These results suggested a broader role of TLKs and ASF1s across various genetic subtypes. Of note, TLK1, TLK2, and ASF1A transcript levels were not significantly different across patients with AML (data not shown) but increased at protein level (Figure 1E). Together, these results indicated that patients with AML have aberrant protein expression of TLKs and ASF1s.
IL-1β–mediated regulation of TLK-ASF1 pathway in AML
We observed that IL-1β stimulation upregulated ASF1B transcript levels in AML progenitors (Figure 1A,C; supplemental Figure 2A). We sought to assess whether IL-1β directly upregulates TLK1 and TLK2 mRNA expression (supplemental Figure 2A). Although there was no increase in the expression of TLK1, an increasing trend for TLK2 transcripts upon IL-1β stimulation was observed. TLK1 and TLK2 levels are upregulated in AML at the protein level upon IL-1β stimulation. Additionally, consistent with increased TLK1 and TLK2 levels, ASF1B and pASF1A levels are increased (Figure 1E; supplemental Figure 2B). Our previous study showed that IL-1β promoted AML cell growth through the activation of the downstream p38MAPK.7 Next, we determined whether p38MAPK regulated the TLK-ASF1 pathway in response to IL-1β. Blockade of p38MAPK (doramapimod or ralimetinib) abolished IL-1β–induced ASF1B expression at mRNA levels, as compared with vehicle-treated AML samples (supplemental Figure 2A). Blockade of p38MAPK did not alter TLK1 or TLK2 expression with and without stimulation of IL-1β (supplemental Figure 2A-B). These results suggest that ASF1B is downstream of IL-1β and p38MAPK. Enrichr analysis30 for the ChIP enrichment analysis data set, using genes that are upregulated by IL-1β in AML progenitors (Figure 1B), suggested that ASF1B may be regulated by several transcription factors including E2F4, FOXM1, MYBL2, and E2F1, the known regulators of ASF1B31 (supplemental Figure 2C; supplemental Table 5). Published chromatin immunoprecipitation sequencing data analysis32 showed that E2F4, FOXM1, MYBL2, and E2F1 occupy the ASF1B promoter (supplemental Figure 2D), suggesting a direct regulation. Analysis of The Cancer Genome Atlas data indicated that the expression of both E2F1 and MYBL2 strongly correlated with high ASF1B expression in patients (supplemental Figure 2E).33 Similar to ASF1 and TLK genes, many AML cell lines showed a high dependency on MYBL2 and E2F1 expression (supplemental Figure 2F).28 Blockade of p38MAPK phosphorylation abolished IL-1β–mediated expression of MYBL2 and E2F1, similar to ASF1B (supplemental Figure 2G). Together, these data suggest that IL-1β may upregulate ASF1B through multiple transcription factors, which is downstream of p38MAPK.
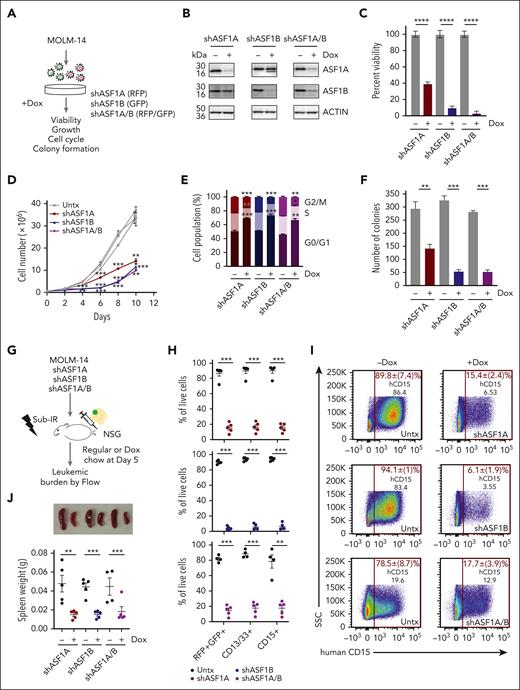
The absence of ASF1 suppresses the growth and leukemia burden of AML
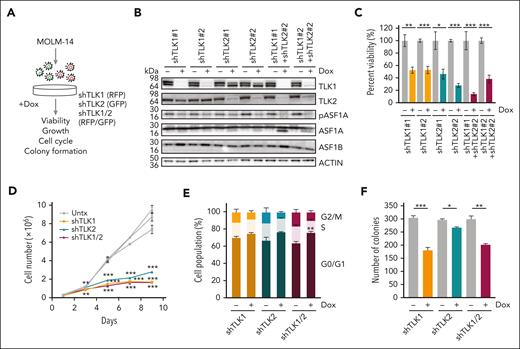
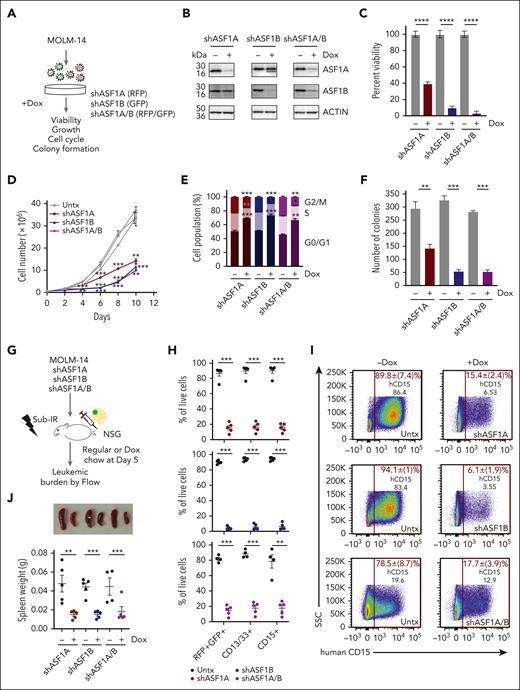
To determine the role of ASF1 in AML, we depleted ASF1A, ASF1B, or both, using doxycycline-inducible, shRNAs in MOLM-14 cells, which harbor FLT3-ITD mutation, one of the most common mutations in AML (Figure 2A-B). Depletion of ASF1A or ASF1B impaired MOLM-14 viability and cell growth (Figure 2C-D). The effect of the combined knockdown of both ASF1A and ASF1B was similar to the knockdown of ASF1B alone, suggesting that ASF1B may compensate for the loss of ASF1A in ASF1A-depleted cells (Figure 2C-D). In addition to MOLM-14 cells, we also depleted ASF1A, ASF1B, or both proteins in MONOMAC-1 cells. We observed reduced viability (supplemental Figure 3A-B) upon the knockdown. However, no changes were observed using nontargeting hairpins (supplemental Figure 3B-D). Cell cycle analysis demonstrated that a proportion of cells accumulated at G0/G1 phase, accompanied by decreased S and G2/M phase populations upon knockdown of ASF1A, ASF1B, or both, suggesting impaired progression from G0/G1 to S phase or cell cycle exit (Figure 2E). Consistent with proliferative defects and cell cycle arrest, the depletion of ASF1A, ASF1B, or both, resulted in a 2.5- to 5-fold reduction in colony formation assay compared with controls (Figure 2F). To examine the effect of ASF1 depletion in vivo, MOLM-14 cells expressing shASF1A (red fluorescent protein–positive [RFP+]), shASF1B (green fluorescent protein–positive [GFP+]), and shASF1A/B (GFP+RFP+) were xenografted into NSG mice and assessed for leukemic burden (Figure 2G). Using RFP/GFP and MOLM-14 cell surface markers CD13+CD33+ and CD15+, we found that ASF1 knockdown reduced the leukemic burden by 80% to 90% in the bone marrow compared with controls (Figure 2H-I). Spleen weight in the doxycycline-treated group was reduced by 80% compared with controls (Figure 2J). MOLM-14 cells expressing nontargeting shRNA (shNT16 or shNT17) were xenografted into NSG mice, and we observed no significant differences in leukemic burden in doxycycline-treated and untreated groups (supplemental Figure 3E-F). Similarly, we found a reduction in the viability of primary AML cells upon ASF1A and ASF1B knockdown (supplemental Figure 3G). Collectively, these results indicate that ASF1A or ASF1B can potentiate AML cell growth and that targeting either was sufficient to impair AML growth.
Reduced ASF1 levels suppressed in vitro and in vivo AML growth and leukemia burden using human cells. (A) A schematic representation of a doxycycline-inducible shRNA model targeting ASF1A, ASF1B, or both ASF1A and ASF1B in MOLM-14 cells. (B) MOLM-14 cells were treated with 1 μg/mL doxycycline to induce knockdown for 6 days. The effect of the knockdown was measured by immunoblotting analysis. shRNA expressing cells were kept in doxycycline-containing media throughout the culture. (C) Cell viability of MOLM-14 cells expressing shASF1A, shASF1B, or both was measured by colorimetric (MTS) assays after 6 days of doxycycline-induced knockdown. (D) Cell growth over time of MOLM-14 cells expressing shASF1A, shASF1B, or both was indicated by cell counts. (E) Cell cycle analysis of MOLM-14 cells expressing shASF1A, shASF1B, or both using propidium iodide and flow cytometry after 3 days of doxycycline-induced knockdown. (F) Colony formation assay was assessed by plating MOLM-14 cells expressing shASF1A, shASF1B, or both in the methylcellulose-based medium following doxycycline-induced knockdown for 14 days. (G) MOLM-14 cells expressing shASF1A, shASF1B, or both were xenografted into sublethally irradiated NSG mice, which were randomly divided into 2 groups (n = 4-5 per group) with or without 625 mg/kg doxycycline chow. All the mice were euthanized 18 days after treatment. (H-I) The leukemic burden was determined by GFP and/or RFP positivity, human CD13/CD33, and CD15 positivity using flow cytometry. (J) Spleen weight and representative images of the spleens are shown from each group. Data are expressed as means ± SEM and the Student t tests were used for statistical analyses. ∗∗P < .01 and ∗∗∗P < .001.
Reduced ASF1 levels suppressed in vitro and in vivo AML growth and leukemia burden using human cells. (A) A schematic representation of a doxycycline-inducible shRNA model targeting ASF1A, ASF1B, or both ASF1A and ASF1B in MOLM-14 cells. (B) MOLM-14 cells were treated with 1 μg/mL doxycycline to induce knockdown for 6 days. The effect of the knockdown was measured by immunoblotting analysis. shRNA expressing cells were kept in doxycycline-containing media throughout the culture. (C) Cell viability of MOLM-14 cells expressing shASF1A, shASF1B, or both was measured by colorimetric (MTS) assays after 6 days of doxycycline-induced knockdown. (D) Cell growth over time of MOLM-14 cells expressing shASF1A, shASF1B, or both was indicated by cell counts. (E) Cell cycle analysis of MOLM-14 cells expressing shASF1A, shASF1B, or both using propidium iodide and flow cytometry after 3 days of doxycycline-induced knockdown. (F) Colony formation assay was assessed by plating MOLM-14 cells expressing shASF1A, shASF1B, or both in the methylcellulose-based medium following doxycycline-induced knockdown for 14 days. (G) MOLM-14 cells expressing shASF1A, shASF1B, or both were xenografted into sublethally irradiated NSG mice, which were randomly divided into 2 groups (n = 4-5 per group) with or without 625 mg/kg doxycycline chow. All the mice were euthanized 18 days after treatment. (H-I) The leukemic burden was determined by GFP and/or RFP positivity, human CD13/CD33, and CD15 positivity using flow cytometry. (J) Spleen weight and representative images of the spleens are shown from each group. Data are expressed as means ± SEM and the Student t tests were used for statistical analyses. ∗∗P < .01 and ∗∗∗P < .001.
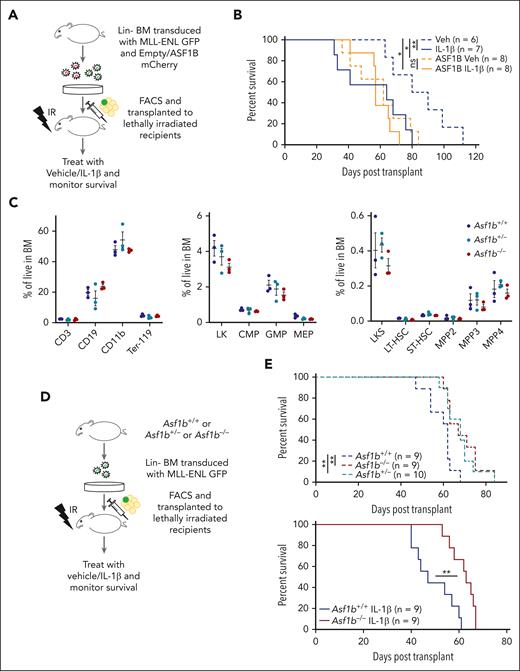
ASF1B is a mediator of IL-1β–driven AML progression
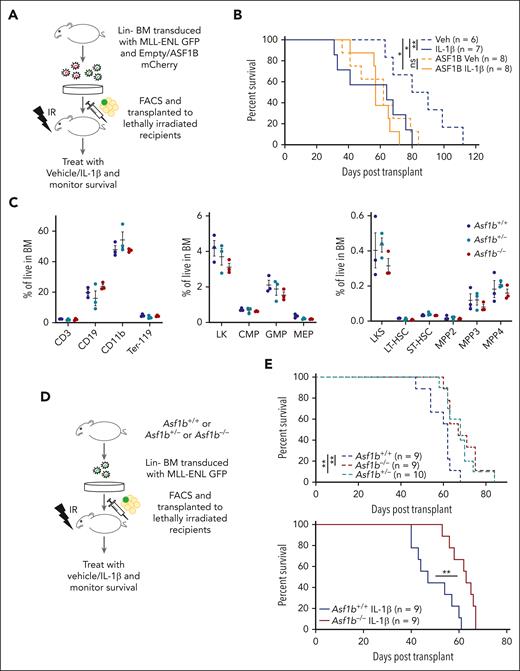
Depmap analysis showed that AML cell lines with broader genetic subtypes were more dependent on ASF1B than ASF1A. Next, to test whether IL-1β promotes AML progression by regulating the expression of ASF1B, we used a murine bone marrow transplantation model of AML driven by an oncogenic fusion protein, MLL-ENL.34 We transduced wild-type bone marrow cells with retrovirus expressing MLL-ENL and GFP, along with retrovirus expression of ASF1B and mCherry, or mCherry alone as an empty-vector control. Sorted double-positive cells were subsequently transplanted into lethally irradiated recipient mice that were treated with IL-1β or vehicle daily via intraperitoneal injection after transplantation (Figure 3A). We found that mice treated with IL-1β succumbed to the disease rapidly (median survival, 64 days; range 31-80 days), compared with the vehicle-treated mice (median survival, 85 days; range, 63-112 days; P = .028). Notably, ectopic ASF1B expression shortened survival (median survival, 62 days; range, 36-84 days; P = .024), mimicking IL-1β–mediated AML progression. However, ASF1B overexpression with IL-1β treatment did not further accelerate disease progression compared with ASF1B alone, possibly because of the saturation of ASF1B levels (Figure 3B). At 5 to 7 weeks after transplant, we observed a higher percentage of leukemic cells in the peripheral blood of ASF1B-overexpressing recipients (supplemental Figure 4A). At the terminal stage, spleen weights were comparable between each group (supplemental Figure 4B) and all the mice showed myeloid expansion of leukemic cells in the bone marrow (supplemental Figure 4C). Taken together, these results suggest that IL-1β treatment or ASF1B overexpression promoted myeloid expansion and leukemia progression. To further evaluate whether ASF1B plays a role in IL-1β–mediated AML progression, we performed complementary studies using Asf1b−/− mice. We first assessed the impact of Asf1b deficiency in normal hematopoiesis and observed no significant difference in differential blood counts of Asf1b+/+, Asf1b+/−, and Asf1b−/− mice (supplemental Figure 4D). Accordingly, flow cytometry analysis of bone marrow cells showed no significant difference in hematopoietic lineages or stem and progenitor cell populations (Figure 3C), suggesting that there are no major hematopoietic defects in Asf1b-deficient cells. Additionally, we performed 1:1 competitive growth assay in vitro, and we observed that Asf1b−/− cells have a slight growth advantage up to day 5 in culture but reduced their growth on day 11. Thus, we did not observe consistent growth differences between Asf1b+/+ and Asf1b−/− cells over time (supplemental Figure 4E). These results are consistent with the immunophenotyping data from these mice (Figure 3C).
ASF1B potentiated IL-1–driven AML progression in a murine model of leukemia, and the absence of ASF1B attenuated the leukemia progression. (A) A schematic representation of a murine AML bone marrow transduction/transplantation model mimicking ASF1B overexpression. Lineage (Lin−)-depleted wild-type bone marrow cells were transduced with MSCV-IRES-mCherry or MSCV-IRES-ASF1B-mCherry and MSCV-IRES-MLL-ENL-GFP constructs. After 48 hours of transduction, 10 000 sorted mCherry+GFP+ cells and supporting cells were injected into lethally irradiated C57BL/6 recipients. After engraftment confirmation, recipient mice were treated with 0.25 μg IL-1β or vehicle and monitored daily. The recipient mice were euthanized when they appeared moribund or showed signs of sickness. (B) Kaplan-Meier survival curve of recipient mice. The log-rank test was used for statistical analysis. ∗P < .05 and ∗∗P < .01 (C) Immunophenotyping of Asf1b+/+, Asf1b+/−, and Asf1b−/− mice representing different cell populations in the bone marrow. The distribution of stem and progenitor cells in the bone marrow of Asf1b+/+, Asf1b+/−, and Asf1b−/− mice was measured by flow cytometry. (D) A schematic representation of a murine AML bone marrow transduction/transplantation model to study the effect of ASF1B deletion on leukemia progression. Lineage (Lin−)-depleted Asf1b+/+, Asf1b+/−, and Asf1b−/− bone marrow cells were transduced with MSCV-IRES-MLL-ENL-GFP retrovirus. After 48 hours of transduction, 25 000 sorted Lin−GFP+ cells and supporting cells were injected into lethally irradiated C57BL/6 recipients. After engraftment confirmation, recipient mice were treated with 0.25 μg IL-1β or vehicle and monitored daily. (E) Kaplan-Meier survival curve of recipient mice. The log-rank test was used for statistical analysis. ∗∗P < .01.
ASF1B potentiated IL-1–driven AML progression in a murine model of leukemia, and the absence of ASF1B attenuated the leukemia progression. (A) A schematic representation of a murine AML bone marrow transduction/transplantation model mimicking ASF1B overexpression. Lineage (Lin−)-depleted wild-type bone marrow cells were transduced with MSCV-IRES-mCherry or MSCV-IRES-ASF1B-mCherry and MSCV-IRES-MLL-ENL-GFP constructs. After 48 hours of transduction, 10 000 sorted mCherry+GFP+ cells and supporting cells were injected into lethally irradiated C57BL/6 recipients. After engraftment confirmation, recipient mice were treated with 0.25 μg IL-1β or vehicle and monitored daily. The recipient mice were euthanized when they appeared moribund or showed signs of sickness. (B) Kaplan-Meier survival curve of recipient mice. The log-rank test was used for statistical analysis. ∗P < .05 and ∗∗P < .01 (C) Immunophenotyping of Asf1b+/+, Asf1b+/−, and Asf1b−/− mice representing different cell populations in the bone marrow. The distribution of stem and progenitor cells in the bone marrow of Asf1b+/+, Asf1b+/−, and Asf1b−/− mice was measured by flow cytometry. (D) A schematic representation of a murine AML bone marrow transduction/transplantation model to study the effect of ASF1B deletion on leukemia progression. Lineage (Lin−)-depleted Asf1b+/+, Asf1b+/−, and Asf1b−/− bone marrow cells were transduced with MSCV-IRES-MLL-ENL-GFP retrovirus. After 48 hours of transduction, 25 000 sorted Lin−GFP+ cells and supporting cells were injected into lethally irradiated C57BL/6 recipients. After engraftment confirmation, recipient mice were treated with 0.25 μg IL-1β or vehicle and monitored daily. (E) Kaplan-Meier survival curve of recipient mice. The log-rank test was used for statistical analysis. ∗∗P < .01.
To determine whether Asf1b deletion suppresses leukemogenesis and IL-1β–mediated progression in vivo, we transplanted Asf1b+/+, Asf1b+/−, and Asf1b−/− cells expressing MLL-ENL and GFP into lethally irradiated recipients (Figure 3D). Heterozygous and homozygous Asf1b deficiency (median survival, 68 and 67 days, respectively; range, 58-84 and 60-84 days, respectively) extended survival of leukemic mice compared with wild-type mice (median survival, 62 days; range, 47-68 days). Notably, Asf1b deficiency attenuated IL-1β–mediated leukemia progression (Figure 3E) (median survival, 63 vs 47 days, respectively; range, 53-67 and 40-61 days, respectively). Additionally, IL-1β–treated Asf1b+/+ mice have shorter survival than vehicle-treated Asf1b+/+ mice (median survival, 47 and 62 days, respectively; P = .006) (supplemental Figure 4F). IL-1β–treated Asf1b−/− mice have slightly shorter survival than vehicle-treated Asf1b−/− mice (median survival, 63 and 67, respectively; P = .046) (supplemental Figure 4F). IL-1β shortened the survival of Asf1b+/+ mice to a greater extent compared with survival of Asf1b−/− mice (Figure 3E). It is possible that other proteins regulated by IL-1β also play roles in AML progression in the absence of ASF1B. At 8 weeks after transplant, we observed a lower percentage of leukemic cells in the peripheral blood of Asf1b+/+ and Asf1b−/− recipients (supplemental Figure 4G). A slight reduction of spleen weights of Asf1b−/− recipients compared with WT was observed (supplemental Figure 4H). Furthermore, the reduced leukemia burden was observed in Asf1b−/− recipients with and without IL-1β treatment (supplemental Figure 4I). Overall, these data suggest that ASF1B is one of the mediators of IL-1β–driven AML progression, and the suppression of ASF1B delayed leukemia progression at a steady state and under inflammatory stress whereas healthy hematopoiesis was not affected.
Genetic targeting of TLK1 and TLK2 suppresses AML growth and leukemic burden
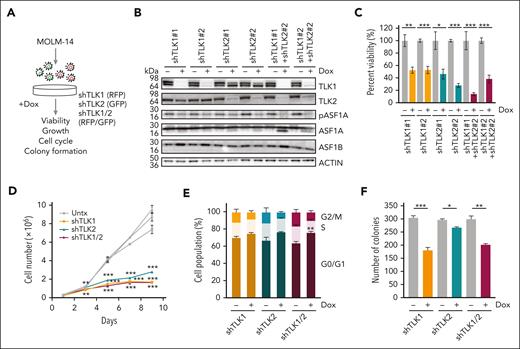
ASF1A and ASF1B are known substrates of the TLKs, which have been proposed to be promising targets for various cancer types.13,14,19 To examine whether TLK1 and TLK2 are also required for AML cell growth and survival, we used doxycycline-inducible shRNA hairpins to knock down TLK1, TLK2, or both, in MOLM-14 cells. As expected, we observed reduced phosphorylation of ASF1A upon knockdown of either TLK1, TLK2, or both. There was no significant change in total ASF1A and ASF1B protein levels (Figure 4A-B). We observed an increased intensity of the lower band for ASF1A upon TLK1 and TLK2, or double knockdown, which might be because the knockdown of both TLKs may reduce levels of phosphorylated ASF1A and the lower band represents a nonphosphorylated form. Similar to ASF1 knockdown and consistent with their role in regulating ASF1 function, depletion of TLK1, TLK2, or both, resulted in a significant reduction of AML cell viability, cell expansion, colony formation ability, and cell cycle progression at the G0/G1 stage (Figure 4C-F). In addition to MOLM-14 cells, we observed reduced viability for TLK2-depleted and TLK1/2-depleted MONOMAC-1 cells and a trend for TLK1-depleted MONOMAC-1 cells. (supplemental Figure 5A). Viability was also reduced in a sample from patient with TLK2-depleted AML (supplemental Figure 5B). Although overexpression of ASF1A was able to rescue the effect of ASF1A knockdown on the viability of MOLM14 cells, a nonphosphorylated form of ASF1A was unable to do so. Interestingly, both wild-type ASF1A and nonphosphorylated form of ASF1A were unable to rescue the effect of TLK2 knockdown on cell viability. These results suggest that ASF1A does not rescue the phenotypes of TLK2 knockdown and 1 possibility is that TLK2 also phosphorylates other substrates. Additionally, TLK1 may also retain its function in TLK2 knockdown cells (supplemental Figure 5C-E).
Reduced expression of TLKs suppresses in vitro and in vivo AML growth and leukemia burden using human cells. (A) A schematic representation of a doxycycline-inducible shRNA model targeting TLK1, TLK2, or both TLK1 and TLK2 in MOLM-14 cells. (B) MOLM-14 cells were treated with 1 μg/mL doxycycline for 6 days to induce knockdown. The effect of the knockdown was measured by immunoblotting analysis. (C) Cell viability of MOLM-14 cells expressing shTLK1, shTLK2, or both was measured by colorimetric (MTS) assay after 6 days of doxycycline-induced knockdown. (D) Cell growth over time of MOLM-14 cells expressing shTLK1, shTLK2, or both was indicated by cell counts. (E) Cell cycle analysis of MOLM-14 cells expressing shTLK1, shTLK2, or both using propidium iodide and flow cytometry after 6 days of doxycycline-induced knockdown. (F) Colony formation was assessed by plating cells in methylcellulose-based medium after doxycycline-induced knockdown for 14 days. Data are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Reduced expression of TLKs suppresses in vitro and in vivo AML growth and leukemia burden using human cells. (A) A schematic representation of a doxycycline-inducible shRNA model targeting TLK1, TLK2, or both TLK1 and TLK2 in MOLM-14 cells. (B) MOLM-14 cells were treated with 1 μg/mL doxycycline for 6 days to induce knockdown. The effect of the knockdown was measured by immunoblotting analysis. (C) Cell viability of MOLM-14 cells expressing shTLK1, shTLK2, or both was measured by colorimetric (MTS) assay after 6 days of doxycycline-induced knockdown. (D) Cell growth over time of MOLM-14 cells expressing shTLK1, shTLK2, or both was indicated by cell counts. (E) Cell cycle analysis of MOLM-14 cells expressing shTLK1, shTLK2, or both using propidium iodide and flow cytometry after 6 days of doxycycline-induced knockdown. (F) Colony formation was assessed by plating cells in methylcellulose-based medium after doxycycline-induced knockdown for 14 days. Data are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
TLK2 is a mediator of IL-1β–driven AML progression
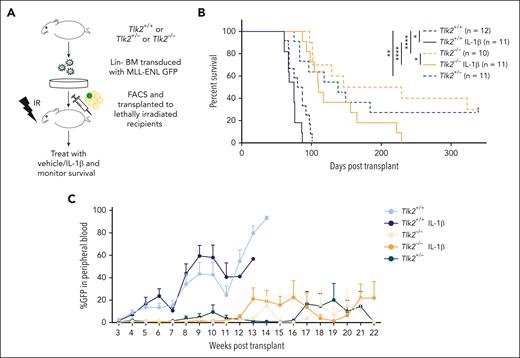
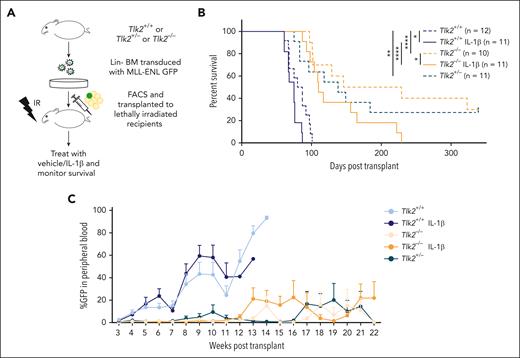
We show that primary AML samples have higher protein levels of TLK1 and TLK2, with greater change in TLK2 than TLK1 levels in AML compared with in healthy cells (Figure 1E). Additionally, the combined knockdown of TLK1 and TLK2 did not confer much stronger growth defects than the TLK2 knockdown alone (Figure 4C-D). CRISPR database analysis showed higher dependency on TLK2 than TLK1 in AML cells (supplemental Figure 1B). Therefore, we asked whether TLK2 loss would impair AML progression. Because complete deletion of Tlk2 is embryonically lethal, we crossed Tlk2fllfl mice with Vav-Cre mice35 to conditionally delete Tlk2 in hematopoietic cells. Vav-Cre Tlk2fl/+ referred to as Tlk2+/−, and Vav-Cre Tlk2fl/fl referred to as Tlk2−/−. Immunophenotyping of Tlk2−/− mice showed slightly lower B-cell numbers than controls but were comparable in other cell types, including stem and progenitor cells (supplemental Figure 6A-C). We did not observe growth differences between CRISPR-edited Tlk2 cells compared with nontargeting cells in competition assays in vitro (supplemental Figure 6D). Using the MLL-ENL murine model of AML (Figure 5A), heterozygous and homozygous deletion of Tlk2 (median survival, 138 days and 187 days, respectively) significantly extended the survival compared with wild-type recipients (median survival, 84 days; P = .006 and P < .0001, respectively). These data suggest that Tlk2 is haploinsufficient for AML progression and even a 50% reduction in expression or activity might improve the survival of the patients. IL-1β treatment promoted AML progression in both wild-type and Tlk2−/− mice compared with vehicle-treated mice (median survival for wild-type, 75 days vs 83 days, P = .033; and median survival for Tlk2−/−, 110 days vs 187 days, P = .046). However, Tlk2 deficiency significantly extended the survival of IL-1β–treated mice compared with IL-1β–treated wild-type mice (median survival, 110 vs 75 days; P < .0001; Figure 5B). Weekly analysis of leukemic burden in the peripheral blood confirmed that heterozygous and homozygous deletion of Tlk2 led to slower leukemia progression compared with wild-type mice (Figure 5C). At the end point, spleen weight and leukemic burden measured by GFP+ cells were comparable between each group (supplemental Figure 6E-F). Furthermore, the flow cytometry analysis of bone marrow cells at the end point showed that Tlk2−/− mice bone marrow cells have reduced lineage-negative cells but that these cells go through apoptosis at a similar rate as Tlk2+/+ cells. The reduced lineage-negative cells might be responsible for the reduced leukemia burden for the mice (supplemental Figure 6G-H). To further understand how TLK2 deletion in the MLL-ENL model affects serial transplantability, we performed a secondary transplantation study. We observed prolonged survival of Tlk2−/− mice (supplemental Figure 6I). However, in contrast to the primary transplant, Tlk2+/− did not extend survival compared with Tlk2+/+ mice, suggesting that efficient targeting of TLK2 is needed to gain effective and long-term effects as a therapeutic target. Taken together, our results showed depletion of TLK2 significantly delayed IL-1β–driven AML progression.
The absence of TLK2 attenuates leukemia burden and IL-1β–driven AML progression in a murine model of leukemia. (A) A schematic representation of a murine AML bone marrow transduction/transplantation model to study the effect of TLK2 deletion on leukemia progression. Lineage-depleted Tlk2+/+, Tlk2+/−, Tlk2−/− bone marrow cells were transduced with MSCV-IRES-MLL-ENL-GFP retrovirus. After 48 hours of transduction, 15 000 sorted Lin−GFP+ cells and supporting cells were injected into lethally irradiated C57BL/6 recipients. After engraftment confirmation, recipient mice were treated with 0.25 μg IL-1β or vehicle and monitored daily. (B) Kaplan-Meier survival curve of recipient mice. The log-rank test was used for statistical analysis. (C) The percentage of leukemic cells in the peripheral blood over time was measured by flow cytometry. ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001.
The absence of TLK2 attenuates leukemia burden and IL-1β–driven AML progression in a murine model of leukemia. (A) A schematic representation of a murine AML bone marrow transduction/transplantation model to study the effect of TLK2 deletion on leukemia progression. Lineage-depleted Tlk2+/+, Tlk2+/−, Tlk2−/− bone marrow cells were transduced with MSCV-IRES-MLL-ENL-GFP retrovirus. After 48 hours of transduction, 15 000 sorted Lin−GFP+ cells and supporting cells were injected into lethally irradiated C57BL/6 recipients. After engraftment confirmation, recipient mice were treated with 0.25 μg IL-1β or vehicle and monitored daily. (B) Kaplan-Meier survival curve of recipient mice. The log-rank test was used for statistical analysis. (C) The percentage of leukemic cells in the peripheral blood over time was measured by flow cytometry. ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001.
Depletion of ASF1A/B or TLK1/2 leads to changes in the protein and phosphoprotein signaling related to the cell cycle and DNA damage response in AML
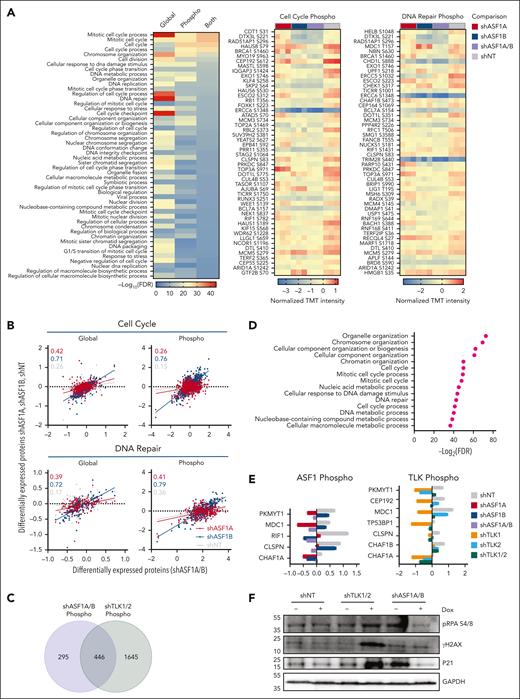
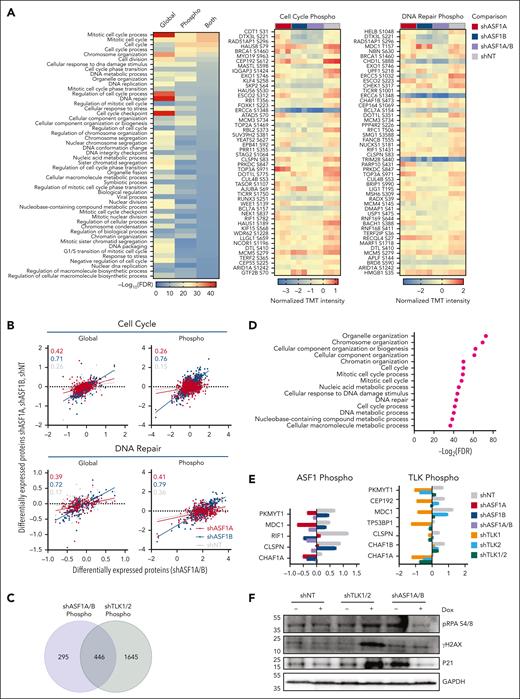
To assess how ASF1s or TLKs deficiency reduced cell growth in vitro and prolonged survival in vivo in AML, we performed global proteomics and phosphoproteomics analyses after the depletion of ASF1s or TLKs in MOLM-14 cells. Upon ASF1A, ASF1B, or ASF1A/B knockdown (supplemental Figure 7A), we observed changes in the levels of 1455, 3335, and 3304 proteins, and 5553, 3699, and 2444 phosphorylation sites, respectively, compared with nontargeting controls (supplemental Table 6). KEGG pathway analysis of altered proteins identified enrichments in the categories of downregulation of the cell cycle, DNA replication, and DNA repair pathways (supplemental Figure 7B). We narrowed our STRING-DB Gene Ontology analysis to ASF1A/B knockdown, which revealed that ASF1A/B knockdown led to decreased protein levels and phosphorylation of proteins associated with the cell cycle (PKMYT1, CEP192) and DNA repair (RIF1, BRIP1, CHAF1B, CLSPN, MDC1) pathways, along with reductions in phosphorylation of DNA repair mediators (TP53BP1, ERCC5) (Figure 6A; supplemental Figure 7B; supplemental Tables 7 and 8). Many of these factors (RIF1, BRIP1, CHAF1B, CLSPN, and MDC1) are consistent with our finding of reduced growth and increased cell cycle arrest in G0/G1 phase upon ASF1A/B knockdown (Figure 2).36-38 Further, several of these factors are known to be associated with ASF1A-mediated changes (MDC1 and RIF1).39,40 However, a more significant correlation was observed between ASF1B and ASF1A/B-related changes than between ASF1A and ASF1A/B (Figure 6B). Upon TLK1, TLK2, or TLK1/2 knockdown in MOLM-14 cells, we observed 1915, 1162, and 3352 differentially expressed proteins, respectively, and 3351, 215, and 8761 changes of phosphorylation sites, respectively (supplemental Table 9). Similarly, the depletion of TLKs resulted in the downregulation of the cell cycle and DNA replication and repair pathways (supplemental Figure 7C). Furthermore, known TLK1 or TLK2 substrates ASF1A-S166, ASF1A-S175, ASF1B-S198, and RAD9-S326 were reduced in TLK1/2-depleted MOLM-14 cells (supplemental Figure 7D).14,41,42 Interestingly, proteins with loss of phosphorylation in TLK1/2 had significant overlap with those with loss in ASF1A/B, 446 of 2091 proteins (Figure 6C). STRING-DB Gene Ontology analysis showed that 446 proteins with decreased phosphorylation levels in both ASF1A/B and TLK1/2 knockdown were enriched in pathways related to cell cycle regulation and DNA repair (Figure 6D; supplemental Figure 8). Specifically, we noted downregulation in the phosphoproteome pathways associated with the cell cycle and DNA repair. We observed phosphorylation of PKMYT1, CEP192, MDC1, TP53BP1, CLSPN, CHAF1B, and CHAF1A were downregulated in TLK1/2-depleted cells with overlapping factors (PKMYT1, MDC1, CLSPN, and CHAF1A) identified in ASF1A/B (Figure 6A,E; supplemental Figure 7E; supplemental Tables 10 and 11). Our immunoblotting data showed that the expression of DNA damage marker yH2AX and cell cycle marker p21 were elevated in TLK1/2-depleted MOLM-14 cells (Figure 6F). Taken cumulatively, the TLK-ASF1 signaling dysregulated the key cell cycle and DNA repair pathways upon ASF1A/B and TLK1/2 depletion, which provides the mechanistic basis for the reduction in cell growth and increased survival of knockout mice in vivo.
Global proteome and phosphoproteome alterations in AML cells upon ASF1 and TLK depletion. Global proteomics and phosphoproteomics analysis of AML cell line MOLM-14 was performed upon knockdown of ASF1s or TLKs as described in supplemental Figure 6A and “Methods.” (A) STRING-DB Gene Ontology analysis for the biological process for proteins that were significantly downregulated for protein levels or their phosphorylation upon ASF1A/B knockdown (left) compared with nontargeting controls (shNT). Analysis of variance (ANOVA) t test, false discovery rate (FDR) <0.05. Additionally, the top 50 downregulated phosphorylation sites for the proteins associated with cell cycle (middle, GO:0007049) and DNA repair (right, GO:0006281) Gene Ontology terms are shown. Scale bar represents normalized TMT intensity using medium centering approach. If multiple phosphorylation sites of the same protein were observed, only the most downregulated phosphorylation sites were shown in the heat maps. (B) The x-axis corresponds to the normalized TMT intensity with medium centering approach of global or phosphorylated protein of all proteins that were significantly altered in shASF1A/B cells (ANOVA t test, FDR < 0.05), whereas the y-axis shows their normalized TMT intensity in shASF1A, shASF1B, or shNT cells. The correlation coefficients (r) are shown for shASF1A/B cells with shASF1A (red), shASF1B (blue), and shNT (gray) cells within cell cycle and DNA repair Gene Ontology terms. (C) Venn diagram showing the overlap of proteins with reduced phosphorylation in MOLM-14 cells with ASF1A/B or TLK1/2 knockdown. (D) STRING-DB Gene Ontology analysis for the biological process for the 446 proteins with reduced phosphorylation in MOLM-14 cells with both ASF1A/B or TLK1/2 knockdown. (E) Representative phosphorylated DNA damage proteins upon ASF1 and TLK knockdown. (F) Immunoblot of DNA damage and cell cycle–related proteins upon ASF1 and TLK knockdown. TMT, tandem mass tags.
Global proteome and phosphoproteome alterations in AML cells upon ASF1 and TLK depletion. Global proteomics and phosphoproteomics analysis of AML cell line MOLM-14 was performed upon knockdown of ASF1s or TLKs as described in supplemental Figure 6A and “Methods.” (A) STRING-DB Gene Ontology analysis for the biological process for proteins that were significantly downregulated for protein levels or their phosphorylation upon ASF1A/B knockdown (left) compared with nontargeting controls (shNT). Analysis of variance (ANOVA) t test, false discovery rate (FDR) <0.05. Additionally, the top 50 downregulated phosphorylation sites for the proteins associated with cell cycle (middle, GO:0007049) and DNA repair (right, GO:0006281) Gene Ontology terms are shown. Scale bar represents normalized TMT intensity using medium centering approach. If multiple phosphorylation sites of the same protein were observed, only the most downregulated phosphorylation sites were shown in the heat maps. (B) The x-axis corresponds to the normalized TMT intensity with medium centering approach of global or phosphorylated protein of all proteins that were significantly altered in shASF1A/B cells (ANOVA t test, FDR < 0.05), whereas the y-axis shows their normalized TMT intensity in shASF1A, shASF1B, or shNT cells. The correlation coefficients (r) are shown for shASF1A/B cells with shASF1A (red), shASF1B (blue), and shNT (gray) cells within cell cycle and DNA repair Gene Ontology terms. (C) Venn diagram showing the overlap of proteins with reduced phosphorylation in MOLM-14 cells with ASF1A/B or TLK1/2 knockdown. (D) STRING-DB Gene Ontology analysis for the biological process for the 446 proteins with reduced phosphorylation in MOLM-14 cells with both ASF1A/B or TLK1/2 knockdown. (E) Representative phosphorylated DNA damage proteins upon ASF1 and TLK knockdown. (F) Immunoblot of DNA damage and cell cycle–related proteins upon ASF1 and TLK knockdown. TMT, tandem mass tags.
Discussion
Despite recent improvements in targeted therapies, the 5-year survival rate for patients with AML has remained low (∼29%) for the past 4 decades.43 This is primarily because of the rapid emergence of drug-resistant mutations in leukemic cells.2 Additionally, the bone marrow microenvironment exerts inflammatory stress and becomes growth permissive to malignant cells over healthy cells. This demands an improved understanding of disease biology and the identification of novel therapeutic targets that are effective across different AML genetic subtypes.
Previously, alterations in the TLK-ASF1 pathway were proposed to contribute to cancer initiation, development, and progression in a variety of cancer types, including breast, colorectal, prostate, melanoma, and cervical squamous cell carcinoma.18,19,44-46 However, the role of the TLK-ASF1 pathway in leukemia and hematopoiesis has not been extensively investigated. In this study we show that aberrant IL-1β signaling drives AML progression by upregulating the TLK-ASF1 histone chaperone pathway. We show that knocking down of either ASF1 or TLK impaired cell growth and survival in both in vitro and in vivo models of AML, representing multiple genetic subtypes of AML. Furthermore, depletion of ASF1B or TLK2 impaired IL-1β–driven AML progression in a murine model, highlighting the potential of targeting the TLK-ASF1 pathway as a novel therapeutic target for AML.
Interestingly, our results indicate that heterozygous or complete deletion of either ASF1B or its regulator, TLK2, delays AML progression at a steady state and under inflammatory stress using IL-1β, suggesting an enhanced dependency of AML on the TLK-ASF1 pathway. Previous work showed that overexpression of CHAF1B, a subunit of the CAF-1 histone chaperone complex that interacts with ASF1B, promoted leukemia progression in a transgenic murine model of MLL-AF9–positive AML.47 Because CAF1 is predominantly involved in replication-dependent histone deposition, these results are consistent with an enhanced dependency of AML on the TLK-ASF1 pathway. However, complete elimination of disease was not achieved upon ASF1B deletion, indicating that other factors, including ASF1A or CAF1, may contribute to AML progression in the absence of ASF1B. This is consistent with our result that ASF1A depletion has a significant impact on AML growth in vitro, and with previous observations that ASF1A depletion caused growth arrest in hepatocellular carcinoma and prostate cancer cells.48 Additionally, we observed 2 bands in some of the ASF1A immunoblots. We suggest that the upper band may be the phosphorylated form of ASF1A because it was abolished by phosphatase treatment according to published studies.39,49 It is possible that ASF1B knockdown may affect ASF1A phosphorylation, or it might be a dominant negative form of ASF1A. However, additional experiments are required to confirm this.
Our proteomics and phosphoproteomics analyses revealed that the TLK-ASF1 pathway promoted leukemogenesis through the regulation of the cell cycle and DNA damage responses, further supporting the TLK-ASF1 pathway's role in promoting AML progression. Moreover, we observed that TLK knockdown had a more significant impact on downstream signaling, as indicated by the modulation of a higher number of phosphorylation sites compared with ASF1 knockdown. Our results also revealed potential new substrates and interacting proteins of TLKs that might be involved in the AML progression.48
To our knowledge, our study is the first to investigate the role of the TLK-ASF1 pathway in inflammation-mediated AML progression. Mechanistically, we found that ASF1B expression is regulated by IL-1β in a p38MAPK-dependent manner, and that transcription factors MYBL2 and E2F1 may be responsible for the transcriptional upregulation of ASF1B.
Importantly, we demonstrated that targeting the TLK-ASF1 pathway effectively reduces leukemia burden and progression in vivo, presenting a promising therapeutic target for AML. Previous work identified the feasibility of targeting ASF1 using a peptide inhibitor that binds to the histone binding pocket and reduces tumor size in vivo.50 Several nonspecific small-molecule inhibitors targeting TLK1 and TLK2 have been tested in the breast cancer models19,51 and recent studies have reported more potent versions of TLK inhibitors,52,53 although their specificity and overall suitability for in vivo use remain unclear.
In summary, our study reveals that chronic inflammation alters the transcriptional landscape of AML and healthy progenitors, thereby driving disease progression. We identified the TLK-ASF1 pathway as a critical mediator in IL-1β–driven leukemia progression and demonstrated that the modulation of this pathway is a novel strategy to prevent inflammatory stress–mediated AML progression without affecting healthy cells. These findings contribute to our understanding of AML biology and provide a foundation for the development of targeted therapies against AML by specifically targeting the TLK-ASF1 pathway.
Acknowledgments
The authors thank all the patients for donating precious samples. RNA quality assessments, library creation, and short-read sequencing assays were performed by the OHSU Massively Parallel Sequencing Shared Resource. The authors also thank the OHSU flow cytometer core, transgenic core, and the OHSU Department of Comparative Medicine and vivarium staff for taking care of the animal colonies. The authors thank Anja Groth for supplying the anti-pS166 ASF1A antibody, and Jeffrey Tyner for sharing AML samples.
Funding for this project was provided by the National Institutes of Health (NIH), National Cancer Institute (NCI) (grant R01 CA229875-01A1); the American Cancer Society (RSG-17-187-01-LIB); V Foundation Scholar award; and the Knight Cancer Research Institute, Oregon Health & Science University as pilot research projects; the Exploratory seed grant award; and Tartar Trust Foundation. A. Agarwal is supported by grants from the NIH, National Heart, Lung, and Blood Institute (NHLBI) (R01 HL155426), Alex's Lemonade Stand/RUNX1 Research Foundation, Edward P. Evans Foundation, Chan Zuckerberg Initiative Funds, as well as NIH, NCI consortia grants U54 CA224019 (S.M., A. Agarwal) and U01 CA257666, and the NIH, NCI’s Office of Cancer Clinical Proteomics Research (Clinical Proteomic Tumor Analysis Consortium) under NIH, NCI grants U01CA214116 and U01CA271412 (S.G., A. Agarwal). H.-Y.L. was supported by a Cancer Biology Stipend from OHSU. M.M. and J.M. are supported by NIH, NHLBI NRSAF31 grants 1F31HL162542 and 1F31HL164052, respectively. M.M. was funded through NIH, NCI T32 grant T32CA254888. T.H.S. was funded by the Spanish Ministry of Science, Innovation and Universities (PGC2018-095616-B-I00/GINDATA and FEDER), received institutional support from the Centres of Excellence Severo Ochoa award, and the CERCA Programme and funding from the Intramural Research Program of the NIH, NCI (ZIA BC 012010). M.V.-P. was funded by an FPI fellowship from the Ministry of Science, Innovation and Universities and support from the IRB Barcelona.
Authorship
Contribution: A. Agarwal provided project oversight for experimental design, data management, data analysis and interpretation, and methods development; A. Agarwal, H.-Y.L., M.M., J.M., M.V.-P., S.J., D.B., C.-F.T., C.P., J.J., A. Adey, S.G., T.L., S.M., and T.H.S. helped with workflow development, experiments, data processing, management, and analysis; and all authors wrote the manuscript and provided feedback.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anupriya Agarwal, Knight Cancer Institute, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, KR-HEM, Portland, OR 97239; email: agarwala@ohsu.edu.
References
Author notes
The accession number for RNA-seq data sets reported in this paper is: phs001657.v2.p1.
All analysis was done using R and the leapR package, which can be found here: https://github.com/PNNL-CompBio/leapR. Further information can be obtained from, and requests for resources and reagents should be directed to and will be fulfilled by, the corresponding author, Anupriya Agarwal (agarwala@ohsu.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.