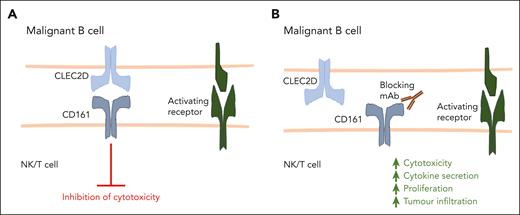
In this issue of Blood, Alvarez Calderon et al develop a fully human monoclonal antibody (mAb) that disrupts the interaction between the inhibitory receptor CD161 and its ligand CLEC2D.1 CD161 blockade enhanced natural killer (NK) cell and T-cell effector function against malignant B cells (see figure), highlighting the potential for targeting CD161 as a novel immunotherapeutic strategy in hematological malignancies.
CD161-blocking antibodies promote cytotoxic lymphocyte function. (A) NK cells, CD4+ T cells, and CD8+ T cells can express CD161, and upon binding with its ligand CLEC2D, CD161 inhibits lymphocyte activation against malignant cells. (B) A fully human, high-affinity CD161-blocking antibody developed by Alvarez Calderon et al disrupts the interaction between CD161 and CLEC2D. This results in enhanced NK cell– and T cell–mediated cytotoxicity against malignant B cells, as well as increased T-cell cytokine production, proliferation, and tumor infiltration.
CD161-blocking antibodies promote cytotoxic lymphocyte function. (A) NK cells, CD4+ T cells, and CD8+ T cells can express CD161, and upon binding with its ligand CLEC2D, CD161 inhibits lymphocyte activation against malignant cells. (B) A fully human, high-affinity CD161-blocking antibody developed by Alvarez Calderon et al disrupts the interaction between CD161 and CLEC2D. This results in enhanced NK cell– and T cell–mediated cytotoxicity against malignant B cells, as well as increased T-cell cytokine production, proliferation, and tumor infiltration.
CD161 is encoded by the KLRB1 gene and is expressed by NK cells as well as tumor-infiltrating CD4+ and CD8+ T cells.2,3 The ligand for CD161, CLEC2D (also named lectin-like transcript), is highly expressed by germinal center (GC) B cells,4 and its expression is further increased by activation signals such as Toll-like receptor signaling and B-cell receptor ligation.5 Importantly, CLEC2D is also expressed by GC-derived non-Hodgkin lymphoma cells, including Burkitt lymphoma, follicular lymphoma, and diffuse, large B-cell lymphoma cells.6 In addition, CLEC2D is expressed by dendritic cells (DCs) and tumor-infiltrating myeloid cells.5-7 Multiple studies have demonstrated that ligation of CD161 inhibits the effector function of NK cells and T cells3,6,7; however, the development of therapeutic approaches targeting this important inhibitory receptor has lagged other immune checkpoint receptors such as PD-1, LAG-3, and CTLA-4. In this study, Alvarez Calderon et al aimed to determine the role of CD161-CLEC2D interactions in hematological malignancies and to develop novel CD161-targeting mAb with potential clinical utility.
Alvarez Calderon et al used publicly available RNA-sequencing data sets to reveal that CLEC2D messenger RNA is expressed at significantly higher levels in hematological malignancies compared with solid tumors. High surface expression of CLEC2D was confirmed by flow cytometry on a panel of cell lines derived from hematological malignancies, indicating that CLEC2D can interact with CD161 in this setting. The authors used a yeast display library to generate a fully human high-affinity anti-CD161 antibody that can selectively disrupt CD161-CLEC2D interactions. mAb blockade of CD161 did not induce T-cell activation in isolation; however, disruption of CD161-CLEC2D interactions in coculture assays significantly enhanced T cell–mediated cytotoxicity against malignant B cells. In addition, CD161 blockade increased T-cell proliferation and the secretion of interleukin-2 and interferon γ (IFNγ) during coculture with tumor cells. In accordance with this, CD161 blockade in combination with adoptively transferred T cells prolonged survival in murine models of lymphoma and leukemia. Interestingly, CD161 blockade increased T-cell infiltration and significantly increased activation of both CD4+ and CD8+ T cells in vivo. Furthermore, single-cell RNA sequencing of tumor-infiltrating T cells revealed upregulation of genes associated with T-cell cytotoxicity and tissue residency in mice treated with CD161-blocking antibody compared with controls.
The authors also assessed how disruption of CD161–CLEC2D interactions affects NK-cell function in hematological malignancies using their novel mAb. In accordance with the effects on T cells, CD161 blockade also significantly increased NK-cell cytotoxicity against malignant B-cell lines in vitro. The expression of CD161 on CD4+ T cells, CD8+ T cells, and NK cells indicates that targeting CD161 has the potential to stimulate a broad immune response in patients. This is important because NK cells and T cells possess complementary antitumor functions.8,9 For example, NK cells are sensitized to tumor cells that have downregulated major histocompatibility complex (MHC) expression, which is a major mechanism of resistance to T cells in cancer. Furthermore, NK cells can promote T-cell function via multiple mechanisms, including the recruitment and activation of DC cells and the secretion of IFNγ, which upregulates tumor MHC expression.8
Interestingly, chimeric antigen receptor (CAR)-T cells have recently been shown to upregulate CD161 expression, in addition to other NK cell–associated inhibitory receptors, following continuous antigen exposure.10 Although not addressed in this study, this indicates that targeting CD161 may have value in the promotion of CAR-T cell function against hematological malignancies. Whether this would be best achieved via mAb or CRISPR-Cas9-mediated silencing of CD161 expression, however, is unclear, although CRISPR-Cas9-mediated silencing of CD161 has previously been shown to improve T-cell function against glioma cells.7 Furthermore, whether CD161 blockade can potentiate the activity of CD20 × CD3 bispecific antibodies against lymphoma cells would be interesting to pursue.
Although CD161 blockade was shown to enhance NK-mediated cytotoxicity in vitro,1 it will be important to test this in vivo using appropriate murine models. In addition, NK cells also contribute to the efficacy of the anti-CD20 antibodies rituximab and obinutuzumab via their expression of FcγRIIIa. CD161-blocking antibodies may therefore have utility in the promotion of antibody-dependent cellular cytotoxicity in combination with anti-CD20 mAb to deepen responses in patients with B cell non-Hodgkin lymphoma.
In conclusion, this important study by Alvarez Calderon et al demonstrates that CD161-blocking antibodies may be an attractive novel approach to engage a broad anticancer immune response against malignant B cells. Additional preclinical studies are now required to identify potentially efficacious and clinically relevant drug combinations to improve the survival of patients with hematological malignancies.
Conflict-of-interest disclosure: M.D.B. has received research funding from Karyopharm Therapeutics.