In this issue of Blood, Wang et al1 report on their use of a combination of molecular technologies including quantitative polymerase chain reaction (qPCR), PrimeFlow RNA assay, and single-cell RNA-sequencing (scRNA-seq) to delineate the critical role hematopoietic stem cells (HSCs) infected with Epsetein-Barr virus (EBV) in the origin of chronic active EBV (CAEBV) disease. These findings will have major implications for understanding the disease pathogenesis of and developing novel therapeutic strategies for CAEBV disease.
Acquisition of EBV infection frequently occurs soon after birth with minimal clinical consequences, but the infection leads to establishment of a pool of latently infected B cells, kept under strict control by virus-specific CD8+ and CD4+ T cells.2 However, primary EBV infection in young adolescents can lead to severe clinical symptoms with uncontrolled proliferation of CD8+ T cells, which are predominantly directed at EBV-encoded lytic antigens.3 Infrequently, this primary EBV infection can lead to nonresolving chronic viral reactivation, also referred to as CAEBV disease.4 In some individuals, prolonged CAEBV disease can lead to a series of fatal complications including gastrointestinal ulceration, hepatic failure, and hemophagocytic lymphohistiocytosis (HLH).5
The management of systemic CAEBV disease is very challenging. “Cooling” therapy incorporating steroids, cyclosporine, and etoposide is typically given, and this is frequently followed by combination chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) or ESCAP (etoposide, cytarabine, l-asparaginase, methylprednisolone, and prednisolone).6 Although responses are common, they are frequently only partial or transient and should be seen as a bridge to what currently remains the only curative option, that is, allogeneic hematopoietic stem cell transplantation (alloHSCT). Indeed, the 3-year overall survival rate of 1 large Japanese series not in receipt of alloHSCT was 0%.7 Wang et al’s findings that EBV is present within HSCs indicate that the reconstitution of non–EBV-infected donor HSCs within the recipient may be the major mechanism of action for alloHSCTs.
Although earlier studies on CAEBV have revealed the identity and phenotypic characteristics of lymphocyte subsets infected with EBV, the precise origin and etiology of this disease remain largely unresolved. EBV infection of B cells through the interaction of viral glycoprotein gp350 and cellular receptors, including CD21 and CD35, is well understood; however, the presence of EBV-infected T cells and natural killer (NK) cells in CAEBV disease raises intriguing questions regarding the precise underlying mechanism for this atypical infection. This issue is particularly relevant as NK cell–associated CAEBV disease often transforms into aggressive malignant disorders such as NK cell leukemia or extranodal NK/T-cell lymphoma. Recent studies have proposed that the origin of these atypical infections may be linked to EBV infection of progenitor lymphoid cells; however, definitive evidence has not been established.8
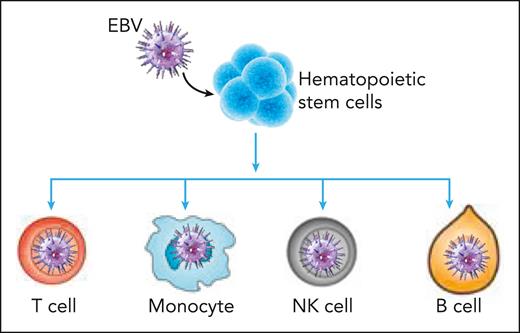
Wang et al explored this issue by analyzing bone marrow and peripheral cells derived from 5 patients with CAEBV disease, 1 patient with EBV-hemophagocytic lymphohistiocytosis (HLH), and 2 control donors. First, they used qPCR and PrimeFlow RNA to detect EBV-infected cells in peripheral blood mononuclear cells (PBMCs) and bone marrow samples. These assays showed EBV-infected cells were primarily enriched in CD56+ NK/NK T cells but also included CD19+ B cells and CD14+ monocytes in both the PBMC and bone marrow. Interestingly, they detected a much higher number of EBV-infected cells in the bone marrow than in peripheral blood. Further analysis of Lin–CD34+ bone marrow cells with PrimeFlow assay confirmed EBV-infected HSCs in 2 patients with CAEBV disease. They further extended this analysis by using scRNA-seq for PBMC and bone marrow samples from patients with CAEBV disease and simultaneously enriched EBV transcripts EBER1, EBER2, EBNA2, EBNA1, and ZEBRA from the same cells for sequencing. Remarkably, this analysis revealed EBV infection in all the hematologic cell populations as well as the stem and progenitor cells. Tracing along the stem cell differentiation tree, the authors observed that all lineage branches were EBV-infected, suggesting that the infection was propagated from the stem cell compartment. Further scRNAseq analysis of PBMCs and bone marrow cells from 1 patient with EBV-HLH and the 2 healthy donors showed significantly fewer infected-cells than in patients with CAEBV disease.
Molecular analysis of bone marrow cells also revealed that EBV infection of HSCs may drive these cells to produce more differentiated progeny, without enlarging the HSC population size (see figure). Indeed, comparative analysis of signature scores calculated based on upregulated genes during cellular differentiation in bone marrow showed higher differentiation rates of infected HSCs but not the differentiation rate of infected multipotent progenitors. This finding was likely the cause of the increasing infection levels along the hematopoietic differentiation pathways. The authors of this study also observed a reduced apoptosis signature and an augmented proliferation score in infected cells. These infected cells showed upregulated expression of EBV genes when compared with the healthy samples. These observations are directly relevant for disease pathogenesis, which is associated with enhanced inflammation and likely contributes to dysregulation of T cell–mediated immune control. The authors also observed lineage-specific differentially expressed genes between infected and uninfected cells, which were related to viral gene expression in T cells and monocytes and cell chemotaxis in neutrophils.
EBV infection of hematopoietic stem cells drives these cells to produce progeny along the differentiation hierarchy, leading to expansion of EBV-infected monocytes, T cells, NK cells, and B cells in CAEBV disease.
EBV infection of hematopoietic stem cells drives these cells to produce progeny along the differentiation hierarchy, leading to expansion of EBV-infected monocytes, T cells, NK cells, and B cells in CAEBV disease.
Finally, 1 of the patients with CAEBV disease had an allogeneic transplant, and 6 months later the authors repeated the assays for EBV copy number and viral gene expression at the tissue and the single-cell levels. This patient showed no detectable EBV DNA in the peripheral blood, bone marrow, and plasma. Single-cell analysis also confirmed the elimination of EBV from hematopoietic system, further supporting the authors’ argument that EBV infection of HSCs contributes to the pathogenesis of CAEBV disease.
Although the data presented by Wang et al provide key insights into the origin of CAEBV disease, these observations will need validation in a larger cohort of patients. One major unanswered issue is how EBV is able to gain entry into HSCs. Based on previously published studies, the authors hypothesize that transfer of messenger RNA from EBV-infected cells via exosomes or direct transfer of viral episomes may contribute to this atypical infection.9,10 Follow-up studies on the mechanism of EBV infection in HSC will provide greater understanding of pathogenesis of CAEBV disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.