Introduction
Patients with relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) are characterized by a particularly poor prognosis. Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy has improved outcomes of patients with r/r DLBCL, yet a substantial proportion of patients still experiences relapse or progression after CAR T-cell treatment. Identifying patients at high risk of CAR T-cell therapy resistance or future lymphoma progression remains challenging. Here, we explored the value of circulating tumor DNA (ctDNA) as a prognostic biomarker at baseline and early into treatment in DLBCL patients undergoing standard-of-care CAR T-cell therapy, without the need for matched tumor genotypes. We further investigated associations between ctDNA and other known clinical risk factors.
Methods
We applied targeted next-generation sequencing (NGS) to a total of 53 samples from r/r DLBCL patients receiving CAR T-cell therapy (axicabtagene ciloleucel and tisagenlecleucel, n=16) at the University Medical Center Freiburg (Germany), using a modified version of the AVENIO ctDNA analysis workflow (Roche; Research Use Only) that covered ~314kb and targeted 466 distinct genes recurrently mutated in DLBCL (based on the CAPP-Seq workflow, Kurtz DM et al. J Clin Oncol 2018). Tumor genotypes were assessed noninvasively from plasma samples obtained at baseline before lymphodepletion with matched germline controls. Concentrations of ctDNA were quantified at baseline and early after CAR T-cell therapy at day 7-10. Initial treatment response was assessed by conventional PET-CT obtained 4-6 weeks after treatment based on the Lugano criteria.
Results
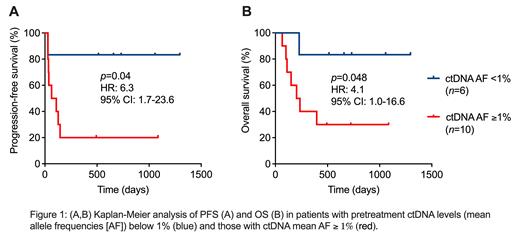
At baseline prior to lymphodepletion, we detected somatic mutations in 100% of plasma samples by noninvasive genotyping, with a median of 19.5 mutations per patient (range: 3-97). The most frequently mutated genes were IGHV, IGLL5, BCL2, MYC, BTG1, CREBBP, and TP53. Patients with high baseline ctDNA concentrations (mean allele frequency [AF] >= 1%) showed shorter progression-free survivial (PFS) and overall survival (OS) than patients with ctDNA levels below this threshold (median PFS: 86.5 days vs. not reached, p=0.04, HR: 6.3, 95% CI: 1.7-23.6; median OS: 219 days vs. not reached, p=0.048, HR: 4.1, 95% CI 1.0-16.6, Figure 1). Pretreatment ctDNA levels were significantly correlated with LDH concentrations ( p=0.049, r=0.5). We further found significantly higher ctDNA concentrations in patients with transformed DLBCL (vs. non-transformed, p=0.008) and in those with 3 or more prior treatment lines (vs. 1-2, p=0.04). At day 7-10 after CAR T-cell therapy, ctDNA levels dropped a median of 10-fold compared to the pretreatment time point ( p=0.008). Patients achieving a complete (CMR) or partial metabolic response (PMR) by PET-CT after 4-6 weeks showed a significant decrease of ctDNA ( p=0.001), while those with a stable (SD) or progressive disease (PD) had no significant ctDNA reduction at day 7-10 after CAR T-cell infusion. Vice versa, while 100% of patients with a >1.5-log-fold drop of ctDNA level between the pretreatment and interim time point revealed a CMR or PMR in early PET-CT scans, only 62% of patients with a smaller ctDNA decline responded to treatment according to PET-CT.
Conclusions
Our data suggests, together with previously published studies (Sworder BJ et al., Cancer Cell, 2023; Frank MJ et al. J Clin Oncol, 2021), that ctDNA profiling may allow robust noninvasive genotyping, accurate risk-stratification, and early prediction of clinical outcomes in r/r DLBCL patients undergoing CAR T-cell therapy. Although limited by small sample size, this pilot study highlights a potential future role of ctDNA measurements for personalized treatment selection and guidance in clinical trials.
Disclosures
Bandara:Roche: Current Employment. Woestmann:Employee of Signature Diagnostics GmbH: Current Employment. Yaung:Roche: Current Employment, Current equity holder in publicly-traded company. Wäsch:Sanofi: Honoraria; BMS: Other: Travel support; Pfizer: Other: Travel support; Kite/Gilead: Other: Travel support; Janssen: Other: Travel support; Takeda: Honoraria; Pfizer: Honoraria; Kite/Gilead: Honoraria; Janssen: Honoraria; BMS/Celgene: Honoraria; Amgen: Honoraria; Abbvie: Honoraria; Sanofi: Research Funding; Janssen: Research Funding; Takeda: Consultancy; Sanofi: Consultancy; Pfizer: Consultancy; Kite/Gilead: Consultancy; Novartis: Consultancy; Janssen: Consultancy; BMS/Celgene: Consultancy; Amgen: Consultancy. Finke:Roche: Current holder of stock options in a privately-held company; Medac: Honoraria, Research Funding; Neovii: Honoraria, Research Funding, Speakers Bureau; Riemser: Honoraria, Research Funding, Speakers Bureau; Gilead Sciences: Current holder of stock options in a privately-held company; AbbVie: Current holder of stock options in a privately-held company. Su:Roche: Current Employment, Current equity holder in publicly-traded company. Scherer:Roche Sequencing Solutions: Research Funding; Gilead Sciences: Research Funding; Takeda: Research Funding.