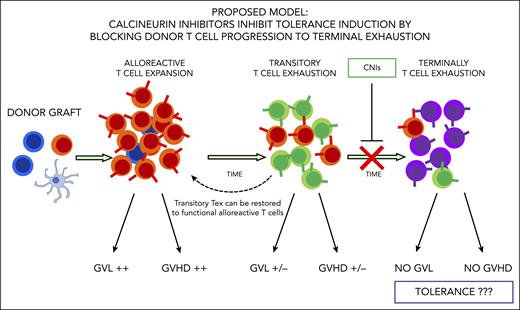
In this issue of Blood, Senjo et al1 show that calcineurin inhibitors (CNIs) suppress donor T-cell progression to terminal exhaustion, thus limiting tolerance induction after hematopoietic stem cell transplantation (HSCT) in a mouse model.
Graft-versus-host disease (GVHD) is still a major cause of transplant-related morbidity and mortality. Prophylactic pharmacologic immunosuppression is required to reduce the incidence and lethality of GVHD after unmanipulated HSCT. CNIs are widely used and are considered the mainstay of posttransplant immune suppression, but, despite their use, acute and chronic GVHD occurs in a large proportion of patients.
Why donor T cells fail to reach a tolerogenic state and induce GVHD in some patients is not well understood. Many studies have focused on identifying the mechanisms of tolerance induction after transplant. Thymic function and the involvement of different donor regulatory cell subtypes (eg, regulatory T cells) have been previously demonstrated to be critically important in the establishment of central and peripheral tolerance.2,3 More recently, the emergence of exhausted donor T cells has been shown to promote tolerance after transplant.4,5 Donor T-cell exhaustion is a multistep process, occurs over time after transplant, and leads to impaired T-cell effector function. It has been described in patients who receive posttransplant pharmacologic immune suppression. Donor T cells that reach exhaustion lose their ability to induce alloreactive responses and have limited ability to induce GVHD but also very little capacity to maintain the desired antileukemic effect.4 Exhausted T cells are still not completely characterized, but they express a combination of inhibitory molecules on their surface (eg, PD-1), upregulate key transcription factors (eg, TOX), and have distinct epigenetic programs.6
How T-cell exhaustion occurs and why this phenomenon differs among patients are still open questions. A better understanding of the mechanisms that support donor T-cell exhaustion might provide relevant insights to improve GVHD prevention and to better balance immune reconstitution after allo-HSCT.
To investigate these issues, Senjo et al explored the reasons why CNIs fail to induce tolerance in a mouse model. The authors found cyclosporine suppresses expression of genes promoting progression to T-cell exhaustion and allows for maintenance of donor T-cell effector function. Expression of genes such as TOX, a master regulator that promotes T-cell progression to “terminally” exhausted T cells, is reduced by treatment with cyclosporine, and T cells are retained in what the authors called a “transitory” exhaustion status. Senjo et al show with elegant experimental models transitory exhausted donor T cells maintain functional alloreactivity. In fact, these cells contributed to the development of chronic GVHD, and their antileukemic activity could be simply restored by PD-1 blockade. Thus, cyclosporine was found to act as a “double-edged” treatment. In fact, it reduces T-cell proliferation and helps protect from acute GVHD occurrence, but, at the same time, it inhibits tolerance induction and plays a key role in the maintenance of an alloreactive state after transplant (see figure).
Donor T-cell exhaustion is considered a major mechanisms of tolerance induction after HSCT. Alloreactive T cells gradually progress to exhaustion after HSCT and lose their ability to induce graft-versus-leukemia (GVL) effect and GVHD over time. CNIs inhibit T cells to reach terminal exhaustion, leaving them in a transitory exhaustion state, thus limiting tolerance induction. Alloreactivity of transitory exhausted T cells can still be restored.
Donor T-cell exhaustion is considered a major mechanisms of tolerance induction after HSCT. Alloreactive T cells gradually progress to exhaustion after HSCT and lose their ability to induce graft-versus-leukemia (GVL) effect and GVHD over time. CNIs inhibit T cells to reach terminal exhaustion, leaving them in a transitory exhaustion state, thus limiting tolerance induction. Alloreactivity of transitory exhausted T cells can still be restored.
Novel insights on how donor T cells differentiate over time is a major contribution by Senjo et al. The authors provide biological evidence of why strategies of posttransplant immune suppression using CNIs fail to induce tolerance. They did show that this failure may actually make it possible to restore antileukemic activity. Such insights are of particular importance taking into consideration the recent availability of different posttransplant cell immunotherapies, from the old-style unmanipulated donor lymphocyte infusions to the new approaches with chimeric antigen receptor T or natural killer cells. Indeed, the previous use of CNIs might strongly influence the persistence of alloreactive T cells and impact the efficacy of such treatments.
The characterization of different T-cell exhaustion states after transplant is also of importance. Identification of transitory or terminally exhausted T cells might help to predict chronic GVHD, and reactivating transitory exhausted T cells could be a promising therapeutic target for a more effective graft-versus-leukemia effect.
Also, graft manipulation strategies that include the infusion of adoptive cell immunotherapies (eg, regulatory and conventional T cells, T-cell receptor α and β/CD19-depleted grafts, CD45RA-depleted T cells, and others) with little or no use of posttransplant pharmacologic immune suppression could be effective alternative approaches that allow for tolerance induction.7,8 Therefore, the study from Senjo et al should spur new studies of donor T-cell progression to exhaustion in using these approaches. Furthermore, in an era in which many new immunosuppressive agents are emerging in the clinical use, the study from Senjo et al reminds us that the impact of such drugs on posttransplant immunity must be carefully evaluated. The best regimen to prevent GVHD or graft manipulation with no or limited use of immunosuppressives depends on many individual patient-related factors.
In conclusion, CNIs will still be widely used, as their safety and efficacy have been proven by years of clinical work. But now we know more about their impact on posttransplant immunity, and there is hope for better pharmacologic manipulations to improve patients’ outcomes.
Conflict-of-interest disclosure: The author declares no competing financial interests.