In this issue of Blood, Matsuoka et al1 revisit the impact of membrane-bound vs soluble KIT ligand (KITL) on hematopoietic stem cell (HSC) regulation in the bone marrow. This study provides a fresh perspective on the prevailing notion that local membrane-bound KITL (mKITL) within the HSC niche is the critical factor, instead proposing that systemic soluble KITL (sKITL) plays a more significant role in HSC homeostasis than previously thought.
HSCs lodge in specialized bone marrow microenvironments, or niches, that tightly control HSC self-renewal, localization, quiescence, and differentiation for replenishment of blood cells via niche regulatory cues in the form of secreted and cell-bound factors. In recent years, the identification and characterization of bone marrow vascular and perivascular niches and their factors supporting HSC function have been under intense investigation.2 However, understanding the relative influence of the local bone marrow niche vs long-range systemic cues from outside the bone marrow on HSC function remains an important and challenging question. Addressing this question is particularly difficult by the lack of tools that allow the discrimination of local from systemic cues. Conditional gene deletions or cellular depletions are valuable approaches, yet their interpretation depends on the specificity of promoter and Cre recombinase excision activity, as well as potential compensatory production of the deleted factor by other cells or long-range soluble signals from outside the bone marrow.
The ligand for the c-KIT receptor on HSCs and progenitor cells, KITL (also known as stem cell factor or steel factor), exists in both a membrane-bound (mKITL) and a soluble form (sKITL).3 Although sKITL is generated through juxtamembrane proteolytic cleavage, mKITL is generated by skipping of the exon that contains the proteolytic cleavage site.4 Early studies using the steel-Dickie (Sld) mutant mice, which express sKITL but lack the mKITL, revealed significant HSC defects,5 suggesting that mKITL is particularly important for HSC maintenance. More recently, conditional deletion of mouse Kitl (required for both mKITL and sKITL expression) in endothelial and leptin receptor (LEPR)+ stromal cells led to reduced HSC numbers in the bone marrow, further supporting the role of KITL in the maintenance of bone marrow HSCs by the local synthesis of mKITL.6 However, new evidence revealed that circulating sKITL levels are also significantly reduced in both genetic models,7 preventing a conclusive determination of the specific roles of local mKITL vs systemic sKITL in sustaining the bone marrow HSCs. In this study, the authors use sophisticated genetically modified mouse models and whole-bone transplant experiments to conclude that systemic sKITL, rather than local mKITL, is key for the maintenance of bone marrow–resident HSCs.
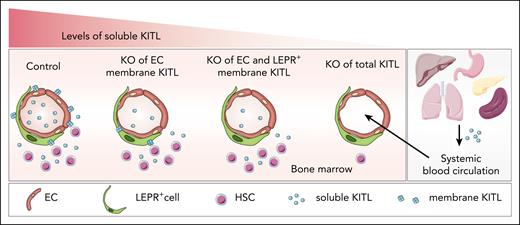
To address the potential distinct role of mKITL and sKITL, the researchers generated a mouse model that selectively depletes mKITL in endothelial cells. Surprisingly, despite a slight reduction in systemic sKITL levels in this model, no significant changes in HSC numbers and function were detected in adolescent and adult mice. Similarly, the combined deletion of mKITL in both endothelial and LEPR+ stromal cells in adult mice had no effect on phenotypical and functional HSCs (see figure). This unexpected finding suggests that in steady-state bone marrow, HSC homeostasis primarily depends on systemic sKITL, which might be produced by endothelial cells or other cells outside the bone marrow niche. Nevertheless, further in-depth analysis is needed to understand how mKITL expression and shedding influence systemic sKITL levels. In addition, more studies are required in the early postnatal bone marrow, as a recent study targeting mKITL in endothelial cells reported a reduction in HSCs.8 However, it is unclear whether sKITL levels were also affected in newborn mice. To further evaluate the role of systemic sKITL, the authors performed elegant renal capsule whole-bone transplant assays. Bones from embryonic day 15.5 (E15.5) wild-type and Sl/Sl embryos (completely lacking expression of sKITL and mKITL) were implanted under the kidney capsule of wild-type mice. Three weeks after transplant, there was no significant difference in phenotypical and functional HSCs in the transplanted bones. This provided strong evidence that systemic sKITL, rather than mKITL, is crucial for the regulation of HSCs in the bone marrow. Intriguingly, outside the bone marrow, specific deletion of mKITL in testicular Sertoli cells, which serve as niche cells for c-KIT+ spermatogonial precursor cells (SPCs), resulted in a significant reduction in c-KIT+ SPCs without affecting systemic sKITL levels, providing strong evidence for the importance of mKITL in the testis. This study's unique approach of selectively targeting mKITL in specific niche cell populations, such as endothelial and testicular Sertoli cells, emphasizes the need for a nuanced understanding of niche cell functions in different tissue microenvironments.
Regulation of bone marrow HSC numbers by soluble KITL. Specific conditional knockout (KO) of mKITL from endothelial cells (ECs) or the combined deletion in ECs and LEPR+ perivascular stromal cells results in a modest reduction in systemic sKITL levels with no significant impact on bone marrow HSCs. In contrast, the deletion of both sKITL and mKITL leads to severe hematopoietic defects. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Regulation of bone marrow HSC numbers by soluble KITL. Specific conditional knockout (KO) of mKITL from endothelial cells (ECs) or the combined deletion in ECs and LEPR+ perivascular stromal cells results in a modest reduction in systemic sKITL levels with no significant impact on bone marrow HSCs. In contrast, the deletion of both sKITL and mKITL leads to severe hematopoietic defects. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
In conclusion, this research provides a fresh perspective on HSC regulation and opens exciting new avenues of exploration of the systemic regulation of HSCs. These findings have unraveled the complex interplay between soluble and membrane-bound KITL in different tissue niches. They also raise questions regarding KITL requirements in the regulation of HSCs during development, stress conditions, and after transplant, where HSC activity and proliferation states differ. More important, this study highlights the need for more specific genetic approaches to identify and understand the functions of distinct niche cells and niche factors within the bone marrow. Furthermore, it suggests a reexamination of the HSC regulatory effects of other known niche factors present in both membrane-bound and soluble forms, such as vascular cell adhesion molecule 1.9 Although the clinical implications of these findings are not directly addressed in this study, the newfound understanding of the regulatory mechanisms underlying HSC maintenance opens exciting possibilities for regenerative medicine.
The potential manipulation of systemic sKITL levels could hold promise in boosting healthy HSC function for improved bone marrow transplant or in the cancer setting, particularly in suppressing therapy resistance associated with activating mutations in c-KIT, as seen in acute myeloid leukemia, where c-KIT inhibitors are being developed for cancer therapy.10
Conflict-of-interest disclosure: S.P. serves as a consultant and has received research funding from Keros Therapeutics. S.Z. declares no competing financial interests.