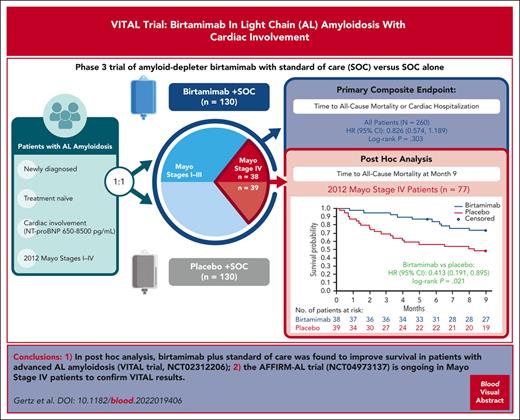
Key Points
The VITAL study of birtamimab in all stages of newly diagnosed AL amyloidosis was discontinued early per futility analysis.
Birtamimab improved post hoc ACM in patients with Mayo stage IV AL amyloidosis with cardiac involvement, who are at high risk of early death.
Abstract
Amyloid light-chain (AL) amyloidosis is a rare, typically fatal disease characterized by the accumulation of misfolded immunoglobulin light chains (LCs). Birtamimab is an investigational humanized monoclonal antibody designed to neutralize toxic LC aggregates and deplete insoluble organ-deposited amyloid via macrophage-induced phagocytosis. VITAL was a phase 3 randomized, double-blind, placebo-controlled clinical trial assessing the efficacy and safety of birtamimab + standard of care (SOC) in 260 newly diagnosed, treatment-naive patients with AL amyloidosis. Patients received 24 mg/kg IV birtamimab + SOC or placebo + SOC every 28 days. The primary composite end point was the time to all-cause mortality (ACM) or centrally adjudicated cardiac hospitalization ≥91 days after the first study drug infusion. The trial was terminated early after an interim futility analysis; there was no significant difference in the primary composite end point (hazard ratio [HR], 0.826; 95% confidence interval [CI], 0.574-1.189; log-rank P = .303). A post hoc analysis of patients with Mayo stage IV AL amyloidosis, those at the highest risk of early mortality, showed significant improvement in the time to ACM with birtamimab at month 9 (HR, 0.413; 95% CI, 0.191-0.895; log-rank P = .021). At month 9, 74% of patients with Mayo stage IV AL amyloidosis treated with birtamimab and 49% of those given placebo survived. Overall, the rates of treatment-emergent adverse events (TEAEs) and serious TEAEs were generally similar between treatment arms. A confirmatory phase 3 randomized, double-blind, placebo-controlled clinical trial of birtamimab in patients with Mayo stage IV AL amyloidosis (AFFIRM-AL; NCT04973137) is currently enrolling. The VITAL trial was registered at www.clinicaltrials.gov as #NCT02312206.
Introduction
Amyloid light-chain (AL) amyloidosis is the most common form of systemic amyloidosis, with an estimated incidence between 8 and 14.4 cases per million person-years.1-4 This rare, typically fatal disease is caused by misfolded kappa (κ) or lambda (λ) immunoglobulin light chains (LCs) from an underlying plasma cell dyscrasia.5,6 Misfolded LC proteins form toxic aggregates and amyloid fibrils that deposit in vital organs, leading to dysfunction,7 most commonly in the heart (80%) and kidneys (66%).8 Cardiac impairment and multiorgan damage are key predictors of reduced survival in AL amyloidosis.1,2,9-11 The mortality risk for newly diagnosed, treatment-naive patients can be categorized using the revised 2012 Mayo Clinic Staging System.12 Mayo stages range from I to IV, with stage IV patients having the highest risk for early mortality (median survival from diagnosis: 5.8 months; 5-year survival rate: 14%).12
Current treatment options for patients with AL amyloidosis target plasma cells in an effort to minimize the production of new LCs.6,13-15 Subcutaneous daratumumab in combination with bortezomib, cyclophosphamide, and dexamethasone is the only US Food and Drug Administration (FDA)-approved therapy for patients with newly diagnosed AL amyloidosis.13,15 However, daratumumab is not approved for the treatment of patients with advanced cardiac AL amyloidosis outside of clinical trials.13,15 Although existing antiplasma cell therapies may provide hematologic responses and partial biomarker-based organ responses, these agents have not demonstrated a survival benefit.6,16-19 There remains a significant unmet need for therapies that can improve survival in patients with advanced AL amyloidosis who are at high risk of early death.12,20
Birtamimab (formerly NEOD001), an investigational humanized IgG1 monoclonal antibody that binds directly to a conserved epitope in misfolded κ and λ LCs, was designed to neutralize toxic soluble LC aggregates and deplete insoluble organ-deposited amyloid via macrophage-induced phagocytosis.21,22 Birtamimab was granted orphan drug status by the US FDA and the European Medicines Agency and received FDA Fast Track Designation.23,24 A phase 1/2 clinical trial in AL amyloidosis patients with persistent organ dysfunction demonstrated that birtamimab is generally safe and well tolerated.25
The phase 3 VITAL clinical trial evaluated the efficacy and safety of birtamimab + standard of care (SOC) vs placebo + SOC in newly diagnosed, treatment-naive patients with AL amyloidosis and cardiac involvement (including N-terminal pro–brain natriuretic peptide [NT-proBNP] ≥650 and ≤8500 pg/mL) by assessing the time to all-cause mortality (ACM) or cardiac hospitalization (CH). When a futility analysis of VITAL was conducted, the independent data monitoring committee recommended discontinuation of the clinical trial, prompting early termination. A numerical trend favoring birtamimab in the primary composite end point for the overall population was observed and was hypothesized to be driven by a treatment effect in the most advanced patients (Mayo stage IV). Thus, further post hoc analyses were performed in patients with Mayo stage IV AL amyloidosis. Here, we present results of the phase 3 VITAL clinical trial, including post hoc analyses conducted in patients with Mayo stage IV AL amyloidosis. Data from these patients served as the basis for the ongoing confirmatory phase 3 AFFIRM-AL study being conducted under a special protocol assessment (SPA) agreement with the US FDA.
Methods
Study design and patients
VITAL was a phase 3, multicenter, global, double-blind, placebo-controlled clinical trial (NCT02312206) conducted between 2016 and 2018 in newly diagnosed, treatment-naive patients with AL amyloidosis and cardiac involvement. The clinical trial was approved by the institutional review boards or ethics committees of all participating sites and was conducted in compliance with the good clinical practice guidelines of the International Council for Harmonization and the principles of the Declaration of Helsinki. Written informed consent to participate in the clinical trial was obtained from all patients.
Adults aged ≥18 years with a biopsy specimen–proven diagnosis of AL amyloidosis were enrolled. Eligibility was determined using either polarizing light microscopy of green birefringent material in Congo red-stained tissue specimens or by characteristic appearance on electron microscopy and confirmation of AL amyloidosis by immunohistochemistry or mass spectroscopy. Additional eligibility criteria included cardiac involvement, defined as follows: (1) past or present clinical signs and symptoms supportive of a diagnosis of heart failure in the absence of an explanation for heart failure other than AL amyloidosis; (2) either an endomyocardial biopsy specimen demonstrating AL amyloidosis or an echocardiogram demonstrating a mean left ventricular wall thickness at diastole >12 mm in the absence of other causes that would adequately explain the degree of wall thickening; (3) NT-proBNP ≥650 and ≤8500 pg/mL; and (4) estimated glomerular filtration rate ≥30 mL/min per 1.73 m2, as estimated using the Chronic Kidney Disease Epidemiology Collaboration equation. Key exclusion criteria were non-AL amyloidosis, meeting the diagnostic criteria for multiple myeloma as per the International Myeloma Working Group, eligibility for and plans to undergo autologous stem cell transplant, or prior treatment with plasma cell–directed chemotherapy. The full VITAL protocol is available online (NCT02312206).
At the last screening visit (day −2 or day −1 before randomization), the severity of AL amyloidosis was determined using the 2012 Mayo Clinic revised staging criteria,12 and the level of renal dysfunction was determined using renal staging criteria26 (supplemental Tables 1 and 2, available on the Blood website). Blood samples were taken for the assessment of hematology and chemistry parameters, including troponin T and NT-proBNP, by the central laboratory. Other assessments included serum free LCs (FLCs), urinalysis, a 6-minute walk test (6MWT), and completion of the short form-36 questionnaire, version 2 (SF-36v2).
Randomization and interventions
Patients were stratified based on the Mayo stage (I/II vs III/IV), renal stage (I vs II/III), and 6WMT distance (<300 vs ≥300 m) and were randomly assigned 1:1 to receive 24 mg/kg (up to a maximum dose of 2500 mg) IV birtamimab + SOC or IV placebo + SOC every 28 days. All patients received concomitant SOC chemotherapy, consisting of a first-line bortezomib-containing regimen administered subcutaneously on a weekly basis, with subsequent plasma cell–directed therapies prescribed as per SOC at the investigator’s discretion. Antiviral prophylaxis was required in patients receiving SOC chemotherapy. Patients who discontinued study drugs were to be followed up until the last adjudicated event.
End points
The primary composite end point was the time to ACM or CH, as centrally adjudicated by the Clinical Events Committee. For ACM, all deaths occurring after the first infusion of study drug until the clinical trial’s final subject’s last visit were included; for CH, all events occurring ≥91 days after the first study drug infusion were included.
Key secondary end points were the change from baseline to month 9 in the SF-36v2 Physical Component Summary (PCS) score, 6MWT distance, and cardiac best response, assessed based on NT-proBNP (supplemental Methods). Safety evaluations included the frequency and severity/seriousness of adverse events (AEs).
Post hoc analyses in patients with Mayo stage IV AL amyloidosis were the time to ACM at month 9 and change from baseline to month 9 in SF-36v2 PCS, 6MWT, and cardiac best response. Hematologic responses better than or equal to a very good partial response (VGPR) by month 3 were assessed in patients with Mayo stage IV AL amyloidosis, defined as a reduction in the difference between involved and uninvolved FLCs (dFLC) to <4.0 mg/dL for patients with baseline dFLC >5 mg/dL. For the post hoc analyses of ACM in patients with Mayo stage IV AL amyloidosis, all adjudicated deaths occurring after the first infusion of the study drug up to month 9 were included. Death records were censored at month 9, given the observed median survival of 8.3 months in the Mayo stage IV placebo group in VITAL and to align with key secondary end points.
Statistical analysis
Efficacy results were analyzed in the intention-to-treat population, which included all randomly assigned patients who received any amount of the study drug and was equivalent to the safety analysis population. For the primary composite end point of time to ACM or CH, the assumed 18-month event rate in the placebo arm was 60%, based on that reported by Kumar et al12 and was assumed to be 42% in the birtamimab arm, corresponding to a hazard ratio (HR) of 0.594. For a two-arm clinical trial with 1:1 randomization and based on the use of a two-sided test at an α = 0.05 level of significance, a total of 156 events (both arms combined) were required for 90% power. The distribution of the primary end point in the 2 treatment groups was summarized using the Kaplan-Meier method. The treatment groups were compared using a two-sided stratified (based on the randomization stratification factors) log-rank test at the α = 0.05 level of significance. Each component of the primary end point was also analyzed.
The SF-36v2 PCS score change from baseline to month 9 was analyzed as prespecified, using a restricted maximum likelihood-based mixed-effect model for repeated measures, including fixed effects for randomization strata, treatment group, categorical time point, and the treatment group × time point interaction, with the baseline value included as a covariate. The 6MWT distance (meters) change from baseline at month 9 was analyzed as prespecified using a rank analysis of covariance model, including fixed effects for randomization strata and treatment group, with the ranked baseline value included as a covariate to address missing data. See supplemental Methods for the ranking of 6MWT distance values. The 6MWT distance (meters) change from baseline to month 9 was analyzed using the same mixed-effect model for repeated measures applied to the SF-36v2 PCS score change estimation.
For the post hoc analyses in patients with Mayo stage IV AL amyloidosis, the same methods were applied as described earlier but only included stratification factors of renal stage (I vs II/III) and 6MWT distance (<300 vs ≥300 m). Sensitivity analyses of ACM in patients with Mayo stage IV AL amyloidosis were also performed, adjusting for key baseline variables. HRs and 90% 2-sided confidence intervals (CIs) were estimated from the semiparametric Cox regression model stratified based on randomization strata (ie, renal stage I vs II/III and 6MWT distance) and with baseline variables including age, sex, race, ethnicity, age at diagnosis, duration since diagnosis, NT-proBNP, dFLC, FLC, New York Heart Association class, troponin T, and 6MWT distance added separately. All baseline variables, except categorical variables (ie, sex, race, ethnicity, and New York Heart Association class), were adjusted as continuous variables. An effect modification analysis comparing HRs of ACM at month 9 was performed to determine whether the observed post hoc treatment effect in patients with Mayo stage IV AL amyloidosis was due to chance. The Cox regression model included treatment (birtamimab vs placebo), Mayo stage (I-III vs IV), and the interaction between treatment and Mayo stage, with stratification factors of renal stage and 6MWT distance. Effect modification of Mayo stage (I-III vs IV) was considered present if the interaction term had a statistically significant P value (P ≤ .05). Number and percentage, along with 2-sided 95% CIs of patients in each category of hematologic response ≥VGPR, are presented by treatment group. A Cochran-Mantel-Haenszel test stratified based on the randomization stratification factors was used to compare the rates of hematologic response ≥VGPR at month 3.
Treatment-emergent AEs (TEAEs) were summarized. The incidence of TEAEs was tabulated based on the system organ class and preferred term for each treatment group as well as severity/seriousness and relationship to the treatment. TEAEs leading to death or study drug discontinuation with grade ≥3 severity and serious TEAEs were summarized and described. TEAEs occurring at any dose that resulted in any of the following outcomes were considered serious: death, life-threatening TEAE, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability/incapacity, congenital anomaly/birth defect, or important medical events. The severity of TEAEs was assessed using the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.0.
Results
Patient disposition and baseline characteristics
A total of 260 patients were randomly assigned, 130 to birtamimab and 130 to placebo, from 70 study sites over ∼2 years. Randomly assigned patients received at least 1 infusion of the study drug and were included in the efficacy and safety analyses. The primary reason for study discontinuation was study termination by the sponsor (148 patients [57%]; supplemental Figure 1).
The baseline demographic and clinical characteristics of all patients in the VITAL clinical trial are summarized in Table 1 based on the treatment group. Patient demographics and baseline disease characteristics were generally well balanced between the birtamimab and placebo groups: the median (quartile [Q] 1, Q3) age at AL amyloidosis diagnosis was 64.1 (57.5, 70.9) and 62.4 (56.8, 69.3) years, time since disease diagnosis was 1.31 (0.92, 1.87) and 1.48 (0.95, 2.17) months, and baseline NT-proBNP was 3146.2 (1650.0, 5173.0) and 3183.7 (1910.0, 5551.0) pg/mL, respectively. Approximately 30% of patients enrolled had Mayo stage IV AL amyloidosis (77 of 260); patient demographics and baseline disease characteristics in this subset of patients were generally balanced between the birtamimab (n = 38) and placebo (n = 39) arms (Table 1).
All patients received concomitant bortezomib-containing chemotherapy regimens; 87.7% of patients received first-line bortezomib, cyclophosphamide, and dexamethasone. SOC regimens given in the second-line setting varied but most commonly consisted of lenalidomide-containing regimens (12.7%). Overall, patients in the birtamimab and placebo arms received a similar median number of study drug infusions, 15.5 (range, 1-35) and 14.0 (range, 1-35) infusions, respectively. The mean (standard deviation) duration of exposure was 389.4 (245.7) days for patients treated with birtamimab and 352.7 (248.3) days for patients administered with the placebo. In the overall population, the median (Q1, Q3) follow-up was 15.7 (12.0, 22.1) months for birtamimab and 14.5 (10.3, 19.8) months for placebo; in patients with Mayo stage IV AL amyloidosis, the median follow-up was 15.2 (9.4, 21.4) and 11.3 (2.3, 17.7) months, respectively.
Efficacy
In the overall population, analysis of the primary composite end point of time to ACM or CH favored birtamimab, but the difference between birtamimab + SOC and placebo + SOC was not statistically significant (HR, 0.826; 95% CI, 0.574-1.189; log-rank P = .303; supplemental Figure 2). Supplemental Table 3 shows results for individual components of the primary end point in the overall population. There were no differences between birtamimab and placebo in the 3 key secondary end points in the overall population (supplemental Table 4).
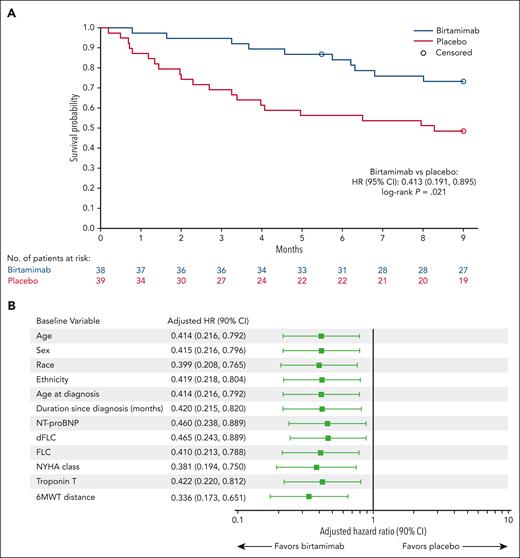
Post hoc analyses of time to ACM in patients with Mayo stage IV AL amyloidosis were subsequently performed to better understand the treatment effect in patients at high risk of early mortality, with survival censored at 9 months, as explained in the methods. The post hoc analysis showed significant improvement in ACM at month 9 for birtamimab + SOC compared with placebo + SOC (HR, 0.413; 95% CI, 0.191-0.895; log-rank P = .021; Figure 1A). The median survival in patients with Mayo stage IV AL amyloidosis was not reached (>9 months) in the birtamimab + SOC arm vs 8.3 months in the placebo + SOC arm. At month 9, the proportion of patients with Mayo stage IV AL amyloidosis surviving was 74% vs 49% in the birtamimab and placebo arms, respectively. Separation of the birtamimab survival curve from the placebo curve occurred early (ie, starting at ∼1 month) and was sustained throughout the study. Sensitivity analyses based on the baseline characteristics helped confirm the robustness of the ACM result in patients with Mayo stage IV AL amyloidosis (Figure 1B). The effect modification analysis comparing HRs for ACM at month 9 between patients with Mayo stage I, II, or III and stage IV AL amyloidosis yielded a statistically significant interaction P value for treatment and Mayo stage (P = .040), suggesting that disease severity at baseline modified the treatment effect of birtamimab.
ACM at month 9 among patients with Mayo stage IV AL amyloidosis. (A) Kaplan-Meier estimate of ACM, with data censored at 9 months and (B) Forest plot of ACM at month 9 adjusted for baseline characteristics in patients with Mayo stage IV AL amyloidosis. HR and 90% 2-sided CIs were estimated from the semiparametric Cox regression model stratified by randomization strata (ie, renal stage I vs II/III, and 6MWT distance), and with baseline variables including age, sex, race, ethnicity, age at diagnosis, duration since diagnosis, NT-proBNP, dFLC, FLC, New York Heart Association (NYHA) class, troponin T, and 6MWT distance added separately. All baseline variables except for categorical variables (ie, sex, race, ethnicity, and NYHA class) are adjusted as continuous variables.
ACM at month 9 among patients with Mayo stage IV AL amyloidosis. (A) Kaplan-Meier estimate of ACM, with data censored at 9 months and (B) Forest plot of ACM at month 9 adjusted for baseline characteristics in patients with Mayo stage IV AL amyloidosis. HR and 90% 2-sided CIs were estimated from the semiparametric Cox regression model stratified by randomization strata (ie, renal stage I vs II/III, and 6MWT distance), and with baseline variables including age, sex, race, ethnicity, age at diagnosis, duration since diagnosis, NT-proBNP, dFLC, FLC, New York Heart Association (NYHA) class, troponin T, and 6MWT distance added separately. All baseline variables except for categorical variables (ie, sex, race, ethnicity, and NYHA class) are adjusted as continuous variables.
The post hoc analysis of SF-36v2 PCS scores for patients with Mayo stage IV AL amyloidosis showed significantly less worsening at month 9 in the birtamimab arm vs that in the placebo arm (least squares mean [standard error]: −0.75 [1.749] vs −5.40 [1.597]; between-group difference, +4.65 [2.325]; P = .046; Table 2). In a post hoc analysis in patients with Mayo stage IV AL amyloidosis, the least squares mean 6MWT distance increased by 15.22 m at month 9 with birtamimab and decreased by 21.15 m with placebo (between-group difference, +36.37 [26.310]; P = .022 from rank analysis of covariance; Table 2). Rank analysis scores for 6MWT distance at month 9 and change from baseline for patients with Mayo stage IV AL amyloidosis are shown in supplemental Table 5. There was no difference between birtamimab and placebo for cardiac best response in these patients as assessed based on the changes in NT-proBNP, a biomarker that to date has not been established as a surrogate end point for product registration (supplemental Table 6). This analysis is limited by missing laboratory data because of early termination of the study. There was no significant difference in the proportion of patients who achieved a hematologic response ≥VGPR by month 3 between treatment arms (relative risk [95% CI], 1.08 [0.56-2.07]; P = .822). In the birtamimab and placebo arms, 12 of 38 patients and 11 of 39 patients, respectively, achieved a hematologic response ≥VGPR.
Safety
Multiple IV infusions of birtamimab were generally safe and well tolerated overall and in patients with Mayo stage IV AL amyloidosis. The rates of TEAEs (all events and serious events) were balanced between treatment groups in the overall population (Table 3). Fatal TEAEs occurred in 15% of patients with birtamimab and 22% of patients with placebo. Consistent with the underlying disease, cardiac disorders were the most common class of fatal TEAEs, occurring in 9 patients (7%) in the birtamimab arm and 18 patients (14%) in the placebo arm. The most common (≥10% of patients in either treatment group) grade ≥3 TEAEs based on preferred term are shown in Table 4, and grade ≥3 TEAEs in the overall population included cardiac failure (birtamimab, 13%; placebo, 20%), pneumonia (birtamimab, 11%; placebo, 9%), and congestive cardiac failure (birtamimab, 10%; placebo, 7%).
In patients with Mayo stage IV AL amyloidosis, all patients reported at least 1 TEAE; serious TEAEs were reported in 27 patients (71%) in the birtamimab group and 29 patients (74%) in the placebo group (Table 3). As with the overall population, serious TEAEs in patients with Mayo stage IV AL amyloidosis were generally assessed by the investigator as unrelated to the study drug. Among patients with Mayo stage IV AL amyloidosis, 4 patients (11%) in the birtamimab group and 14 patients (36%) in the placebo group experienced a TEAE resulting in death. The percentages of patients with grade ≥3 TEAEs were 79% in the birtamimab arm and 90% in the placebo arm, and they were generally assessed by the investigator as unrelated to the study drug (Table 3). The 3 most common grade ≥3 TEAEs in patients with Mayo stage IV AL amyloidosis were syncope (birtamimab, 16%; placebo, 15%), cardiac failure (birtamimab, 13%; placebo, 28%), and congestive cardiac failure (birtamimab, 13%; placebo, 8%) (Table 4).
In the overall population, TEAEs associated with infusions were reported in 5 patients (4%) with birtamimab and 3 patients (2%) with placebo. All infusion-associated TEAEs were nonserious and mild or moderate in severity, except for a grade 3 infusion-related reaction that occurred in a patient who was birtamimab-treated on day 226 and resolved on the same day. All other infusion-associated TEAEs in the birtamimab group occurred on day 1. In patients with Mayo stage IV AL amyloidosis, infusion-associated TEAEs were reported in 3 patients in the birtamimab group and included dyspnea (n = 1), chest discomfort (n = 1), and hypoxia concurrent with an infusion-related reaction (n = 1).
In the overall study population, 41 patients (32%) in the birtamimab arm and 42 patients (32%) in the placebo arm died during the study; except for 1 death in the placebo arm, all were adjudicated by the Clinical Events Committee. Cardiac disorders were the most common cause of death, occurring in 21 patients in the birtamimab arm and 28 patients in the placebo arm of the overall study population, consistent with the underlying disease and the known risk of cardiac complications in AL amyloidosis. Among patients with Mayo stage IV AL amyloidosis, there were 14 (37%) deaths in the birtamimab arm and 22 (56%) in the placebo arm, of which 8 and 15, respectively, were attributed to cardiac events. The largest proportion of adjudicated deaths among patients with Mayo stage IV AL amyloidosis occurred in the first 3 months of the study: 2 patients (5%) in the birtamimab arm and 12 patients (31%) in the placebo arm.
Discussion
To our knowledge, VITAL is the first randomized, placebo-controlled phase 3 trial of an amyloid-depleter therapy combined with SOC chemotherapy in patients with AL amyloidosis with cardiac involvement (NT-proBNP ≥650 and ≤8500 pg/mL). It is unlikely that this study would have been able to detect a difference in survival between treatment groups in patients with Mayo stages I-III AL amyloidosis without a considerably longer duration of treatment, given the reported median survival for patients with Mayo stage I, II, and III AL amyloidosis of ∼94, 40, and 14 months, respectively.12 The primary composite end point of the time to ACM or CH favored birtamimab, although the difference between treatment arms did not reach the prespecified significance. Post hoc analyses in patients with Mayo stage IV AL amyloidosis showed a potential effect of birtamimab on mortality in patients with the highest risk of early mortality. Analyses in patients with Mayo stage IV AL amyloidosis were conducted using the time to ACM at month 9 as the efficacy end point based on median survival in the Mayo stage IV placebo group of 8.3 months and to align with the key secondary end points (change from baseline to 9 months). In patients with Mayo stage IV AL amyloidosis, significant improvement in survival with birtamimab + SOC was observed at month 9 (HR, 0.413; 95% CI, 0.191-0.895; log-rank P = .021). An effect modification analysis confirmed that the severity of disease at baseline affeced the observed treatment effect of birtamimab, which may be attributable to the paucity of events in patients with Mayo stage I-III AL amyloidosis over the duration of the study.
Treatment with birtamimab in patients with Mayo stage IV AL amyloidosis was associated with significantly less deterioration in quality of life (QoL), as measured using SF-36v2 PCS, and improved cardiac functioning, per 6MWT. In this subgroup, treatment with placebo + SOC led to a substantial decline in 6MWT distance over 9 months (∼21 m), whereas distance increased by ∼15 m with birtamimab + SOC during the same period. This suggests, in addition to potentially imparting a survival benefit, birtamimab may also confer a clinically meaningful impact on QoL and functional capacity in patients with advanced disease.
Newly diagnosed patients with Mayo stage IV AL amyloidosis are at high risk of early death, within 6 months of diagnosis (median overall survival, 5.8 months), with cardiac failure being the leading cause of death.6,12,18 Consistent with this previously reported mortality risk, >50% (12 of 22) of the deaths in patients with Mayo stage IV AL amyloidosis treated with placebo + SOC during this clinical trial occurred within the first 3 months. Current SOC in AL amyloidosis consists of repurposed multiple myeloma therapies and is aimed at reducing or eliminating the plasma cell dyscrasia rather than directly depleting existing AL amyloid deposits or targeting toxic soluble LC aggregates.6,13,20 In contrast, birtamimab is a humanized IgG1 monoclonal antibody that directly targets a shared cryptic epitope on misfolded κ and λ immunoglobulin LCs and is designed to neutralize toxic soluble aggregates of misfolded LCs, prevent aggregation of newly produced LCs, and deplete existing insoluble organ-deposited amyloid.21,22 SOC therapies typically require ≥6 months to achieve organ responses, which are evaluated using biomarkers.17 Notably, we observed no difference in the hematologic response rates between treatment arms, which suggests the observed potential survival benefit with birtamimab was not due to a higher hematologic response, consistent with birtamimab’s mechanism of action.
Because patients with Mayo stage IV AL amyloidosis are at the highest risk for early mortality, novel, safe, and effective therapies to rapidly deplete organ-deposited amyloid are urgently needed.12,13,20 To our knowledge, no other investigational or approved therapy has demonstrated a survival benefit in patients with Mayo stage IV AL amyloidosis. Our post hoc analysis was restricted to patients with NT-proBNP between 1800 and 8500 pg/mL; nonetheless, mortality in the placebo arm was generally consistent with historical survival rates in patients with Mayo stage IV AL amyloidosis, who are at high risk for early death.12,27 Furthermore, the post hoc result observed here, with birtamimab + SOC in patients with Mayo stage IV AL amyloidosis (median survival not reached, >9 months), suggests that birtamimab could play a role in achieving an early survival benefit in patients with advanced AL amyloidosis.
Patients with advanced AL amyloidosis are typically frail and have numerous underlying comorbidities, making them less tolerant of SOC.6,20,28 Many SOC therapies are associated with AEs that can worsen patients’ clinical status.13,20 Poor tolerability can lead to treatment discontinuation and detrimentally affect the ability to achieve a robust hematologic response,26,28 highlighting the unmet need for novel therapeutics with a favorable benefit-risk profile for advanced AL amyloidosis. In VITAL, once-monthly IV infusions of birtamimab (median, 15.5 infusions) over a median follow-up of ∼15 months were generally safe and well tolerated, and the safety profile in patients with Mayo stage IV AL amyloidosis was generally consistent with that in the overall study population. In addition, infusion-associated TEAEs occurred with relatively low frequency in 5 patients with birtamimab and 3 patients with placebo; all were mild or moderate with birtamimab and generally occurred early during treatment.
The limitations of post hoc analyses are well known; by nature, they have a greater potential for type 1 error, meaning there is an increased potential for a false-positive result. Thus, the findings from these post hoc analyses should be interpreted with caution. Because of early termination of the trial based on futility analysis, post hoc efficacy analyses were limited to 9 months, and long-term follow-up for survival was not possible. Immunogenicity and pharmacokinetic data from VITAL were not analyzed; however, previous data from the phase 1/2 clinical trial of birtamimab demonstrated a well-behaved IgG1-like pharmacokinetic profile that did not appear affected by underlying renal, cardiac, or neurological involvement and showed no antidrug antibodies among 27 patients who were treated with birtamimab.25 These results are consistent with birtamimab being a humanized monoclonal antibody, given that reducing the amount of nonhuman sequence in monoclonal antibodies has been associated with a decreased risk of immunogenicity.29
Because of the significant survival and clinical benefits observed with birtamimab + SOC in the post hoc analyses of VITAL reported here, a confirmatory global phase 3 randomized, double-blind, placebo-controlled clinical trial of birtamimab in patients with Mayo stage IV AL amyloidosis, AFFIRM-AL (NCT04973137), is being conducted under an SPA agreement with the US FDA.30
Conclusion
The phase 3 VITAL clinical trial was stopped early based on a recommendation from the independent data monitoring committee after the results of a futility analysis that suggested the primary end point was unlikely to be met. Post hoc analyses demonstrated a significant survival benefit with birtamimab and significant improvements in QoL and functional capacity in patients at the highest risk for early mortality (Mayo stage IV). Overall, the incidence, severity, and seriousness of AEs were similar in each treatment group, indicating that birtamimab was generally safe and well tolerated. Given the urgent unmet need for treatments that improve survival in patients with advanced AL amyloidosis, the confirmatory AFFIRM-AL study of birtamimab (NCT04973137) in this patient population is ongoing under an SPA agreement with the US FDA.
Acknowledgments
The authors thank Peloton Advantage, LLC, an OPEN Health company, for their contributions toward initial drafting of the manuscript. The authors thank Martin Koller for his contributions to the VITAL Study design and data acquisition.
Medical writing and editorial support were provided by Emily Mercadante of Amiculum Ltd, funded by Prothena Biosciences Ltd. This clinical trial was sponsored by Prothena Biosciences Inc., Dublin, Ireland, a member of the Prothena Corporation plc group.
Prothena Biosciences Ltd participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Authorship
Contribution: M.A.G, J.W., and G.G.K. designed the study; M.A.G., J.W., S.G., and G.G.K. were responsible for the conduct of the study; C.N. was responsible for data collection; Y.J. performed analysis of data; A.D.C., R.L.C., E.K., H.J.L., E.N.L., M.L., V.S., S.S., A.W., J.A.Z., G.P., and G.M. assisted with patient accrual and data analysis; C.N., C.K., Y.J., G.G.K., and M.A.G. assisted in preparation of the manuscript; and all authors had full access to study data and take responsibility for the integrity of the data and the accuracy of the data analysis and revised the manuscript as well as reviewed and approved the final version for submission.
Conflict-of-interest disclosure: M.A.G. reports personal fees from Alnylam, Aptitude Health, Ashfield, Celgene, Ionis/Akcea, Janssen, Johnson & Johnson, Juno, Physicians Education Resource, Prothena, Research to Practice, Sanofi, and Sorrento; reports personal fees for data safety monitoring board from AbbVie; reports payment for development of educational materials for i3Health and educational program development for i3Health; and received grant funding from NCI SPORE MM SPORE 5P50 CA186781-04. A.D.C. is a consultant for AbbVie, Arcellx, AstraZeneca, BMS, Celgene, Genentech/Roche, GlaxoSmithKline, Ichnos, Janssen, Kite Pharma, Oncopeptides, Pfizer, Seattle Genetics, and Takeda; received research funding from GlaxoSmithKline and Novartis; and holds patents and royalties in Novartis. R.L.C. is a consultant and adviser for Caelum; is a consultant and adviser for and recieved research funding from Janssen and Prothena; received research funding from Karyopharm, and Takeda; is an adviser and received research funding from Unum; and received royalties for patent 9593332, pending 20170008966. E.K. is a consultant for Amgen, Janssen, Genesis, Takeda, Prothena, and Pfizer; and received research funding from Amgen and Janssen. H.J.L. is an adviser for Caelum, Janssen, Prothena, and Pfizer; is a consultant and adviser for Celgene; is a consultant for Karyopharm; is an adviser and received research funding from Takeda; and received research funding from Amgen. E.N.L. is a consultant for AbbVie, Akcea, Alnylam, Pharmacyclics, and Janssen. M.L. received research funding from Alexion, Caelum, and Prothena; and is an adviser for Janssen and Takeda. V.S. is a consultant and adviser for and received research funding from Caleum; is a consultant for Attralus and Pfizer; received research funding from Celgene, Karyopharm, Millennium-Takeda, Oncopeptides, Prothena, and Sorrento; received research funding from and is an adviser for Janssen; and is an adviser for AbbVie, Proclara, Protego, Pharmatrace, Prothena, Regeneron, and Telix. S.S. is an adviser for and received travel grant, honoraria, and research funding from Janssen and Prothena; received research funding from Sanofi; received honoraria from Pfizer and Takeda; and is an adviser for Telix; received travel grants from Binding Site, Celgene, and Jazz. A.W. received research funding from Amgen; and received honoraria from Alexion, Celgene, GlaxoSmithKline, Janssen-Cilag, Prothena, and Takeda. J.A.Z. is a consultant/adviser for Alnylam, Amgen, BMS, Caelum, Celgene, Intellia, Janssen, Oncopeptides, and Takeda; and received research funding from BMS and Celgene. G.P. is an adviser for and received honoraria from Janssen, Protego, and Zentalis; and received honoraria from Pfizer, Sebia, Siemens, and The Binding Site. J.W. is employed by Consulting JW LLC; is a consultant for 4DMT, Aduro/Chinook, Alpha Holdings, Ambagon, Aminex, Arch Oncology, AroBio, Benevolent/BAI, CALIBR – Scripps, Cocept, Crinetics Pharmaceuticals, Crown BioScience, Cumulus Oncology Limited, Cybrexa, CytomX, Dren Bio, Entos, Excure Inc, Flag, Fulgent, Harpoon, Immune Onc, ImmuNext, InClin, January Therapeutics, Janux Therapeutics, Myovant, Nurix, Nuvation Bio Inc, OPNA Bio LLC, Orbus, Orphagen, Oryzon, Plexxikon, Propella, Que Oncology, Sagamore, Sesen Bio, Shape Therapeutics, Sonnet BioTherapeutics, Trex, and venBio Partners LLC; and received payment for expert testimony from Puma Biotechnology. S.G. is the founder of and employed by Attralus Inc; owns stock or stock options in Prothena and Attralus; and is named inventor on patents related to birtamimab and Attralus. C.N., C.K., and Y.J. are employed and stockholders at Prothena. G.G.K. is an employee of and has equity ownership in Prothena; and is a named inventor on multiple patents and patent applications related to birtamimab. G.M. declares no competing financial interests.
The current affiliation for J.W. is Consulting JW LLC, San Francisco, CA.
The current affiliation for S.G. is Attralus Inc, San Francisco, CA.
A complete list of the VITAL Study Investigators appears in the supplemental Appendix.
Correspondence: Morie A. Gertz, Division of Hematology, Department of Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gertm@mayo.edu.
References
Author notes
Subject to certain criteria, conditions, exceptions, and applicable data privacy laws, individual deidentified participant data from Prothena-sponsored global interventional clinical studies conducted for medicines for indications that have been approved are available on request from Prothena (medicalinfo@prothena.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.