Perhaps no more complex issue in clinical immunology exists than the one revolving around allogeneic hematopoietic stem cell transplantation (alloHSCT) and dealing with the tightrope of controlling graft-versus-host disease (GVHD) but still generating needed graft-versus-leukemia (GVL) effects. In this issue of Blood, using different mouse preclinical models, Kim et al1 build off earlier literature demonstrating that absence of interferon gamma (IFN-γ) signaling on donor T cells results in less GVHD by now providing insights on downstream pathways involved.
Fortunately, as we understand more about the immunologic pathways underlying these processes, we can develop better means to potentially separate them. Notably, they demonstrate that impairment of IFN-γ signaling on donor T cells resulted in increased production of the calcium-binding protein, S100a9, which itself had direct suppressive effects on GVHD, yet still allowing for GVL responses. More important, their studies also demonstrated multiple approaches in potentially exploiting this pathway for GVHD suppression in their models. This was achieved by use of blocking IFN-γ receptor antibodies or deleting the IFN-γ receptor to increase S100a9 production, engineering donor T cells to overexpress S100a9, or simply administering S100a9 in vivo, which all led to suppression of acute GVHD.
The separation of GVHD/GVL after alloHSCT has always represented a unique dilemma from the immunologic standpoint, given the mutual processes/cells involved. It centers on the pivotal role of the donor T cell and amplifying inflammatory responses. However, despite the similarity of the immune processes in GVHD/GVL, they also represent opposite ends of the immunologic spectrum regarding management. Treatment or prevention of GVHD generally involves immunosuppression in some form to reduce donor-mediated tissue attack. In contrast, promotion of GVL entails immune stimulation. Therefore, any regimens applied to prevent GVHD must allow for sufficient GVL, and any means to promote GVL should not also promote GVHD. Further complicating the picture, many times, the same cytokine or immune processes can have multiple and sometimes opposing effects in both GVHD/GVL. Case in point: the complex and pivotal role of IFN-γ on GVHD/GVL. IFN-γ produced by donor T cells is well described as a critical mediator for anti-tumor responses, but unfortunately also contributes to GVHD pathogenesis and inflammatory effects.2,3 IFN-γ also directly affects the donor T cells themselves in GVHD and, thus, may be of potential use as a target. Intriguingly, reports on preclinical data from >10 years ago demonstrated that donor T cells from IFN-γ receptor-deficient mice resulted in less GVHD yet still maintain GVL after alloHSCT.4,5 However, the mechanisms underlying these differential effects were not elucidated, and not much else was reported since then.4,5 The present study by Kim et al sheds light on this and demonstrates that at least one pathway that may be responsible is the induction of S100a9, also known as calgranulin or calprotectin by the donor T cells. Interestingly, this molecule has been shown to have numerous, and at times conflicting, effects primarily centering on inflammatory responses.6 Effects on neutrophil homeostasis, macrophage functions, and platelet and vascular inflammation all have been reported as well as roles in infection and autoimmune states. Using several mouse models in which the mouse donor T cells lack the IFN-γ receptor or were engineered to overexpress S100a9, as well as a xenogeneic GVHD model in which antibodies to the human IFN-γ receptor targeted human T cells in vivo, Kim et al demonstrate protection from GVHD and reduced T-cell trafficking to GVHD target organs, such as the gut (see figure). More important, GVL was maintained in the preclinical models. The approaches used in this study provide some intriguing means that could be exploited clinically using both cell engineering as well as direct administration of S100a9.
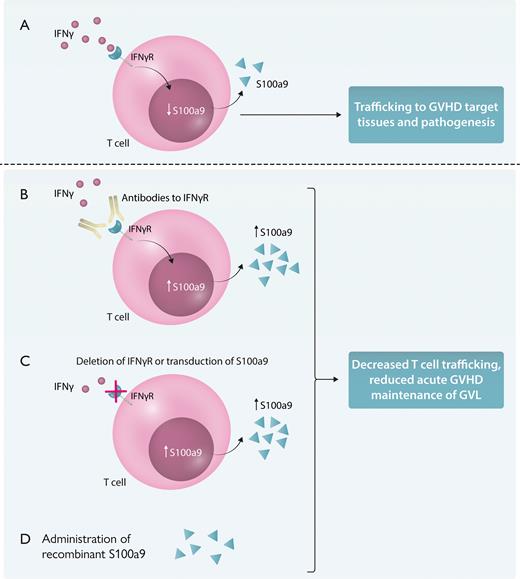
Targeting IFN-γ signaling and S100a9 on GVHD/GVT. (A) IFN-γ signaling on donor T cells reduces S100a9 production and is associated with T-cell trafficking to the gut and GVHD target tissues. Alteration of this pathway by antibody-mediated blockade of IFN-γ receptor binding (B), deletion of IFN-γ receptor on donor T cells, or transduction of S100a9, resulting in overexpression, all of which result in increased production of S100a9 (C), or administration of S100a9 results in reduced T-cell trafficking to GVHD target tissues, such as the gut, and suppresses GVHD while still allowing for GVL (D). IFNγR, interferon-gamma receptor. Professional illustration by Somersault18:24.
Targeting IFN-γ signaling and S100a9 on GVHD/GVT. (A) IFN-γ signaling on donor T cells reduces S100a9 production and is associated with T-cell trafficking to the gut and GVHD target tissues. Alteration of this pathway by antibody-mediated blockade of IFN-γ receptor binding (B), deletion of IFN-γ receptor on donor T cells, or transduction of S100a9, resulting in overexpression, all of which result in increased production of S100a9 (C), or administration of S100a9 results in reduced T-cell trafficking to GVHD target tissues, such as the gut, and suppresses GVHD while still allowing for GVL (D). IFNγR, interferon-gamma receptor. Professional illustration by Somersault18:24.
Interestingly, the role of IFN-γ and S100a9 in inhibiting GVHD had been reported previously because of effects on plasmacytoid dendritic cells.7 This would suggest that both IFN-γ receptor signaling and S100a9 may have effects on multiple cell types outside of the donor T cells, and this may contribute to effects on GVHD/GVL, depending on what targeting approach is applied.
What are the next steps? It will be important to delineate the precise role of S100a9 when blocking or inhibiting IFN-γ receptor signaling on the donor T cells as that may just be one of the pathways involved. Similarly, administration of recombinant human S100a9 needs to be evaluated to confirm the results in the mouse GVHD/GVL studies. It will also be critical to rigorously address potential effects on cancer progression given multiple reports that some cancers can exploit the inflammatory/immunosuppressive effects attributed to S100a9 via myeloid-derived suppressor cells (MDSCs), promoting the progression of a variety of cancers, including chronic myeloid leukemia, breast cancer, and colon cancer.8,9 This would suggest that application needs to be likely tailored for leukemias not directly promoted by it or with an already strong MDSC presence. Although GVL responses were maintained in the present study, the question arises if more subtle effects on tumor progression occur and would even be detected using current mouse GVL models, which have significant limitations. This has been an ongoing issue with preclinical GVHD/GVL studies in general, with the emphasis by far centering on GVHD mitigation. Thus, the tightrope between sufficient GVHD suppression without impairing GVL still exists. Nonetheless, it is encouraging that as we continue to delve deeper underlying the immunology of GVHD/GVL, even building off initial observations from >10 years ago, that more targets of opportunity can be gleaned and potentially used in alloHSCT. Slow and steady can win the race.
Conflict-of-interest disclosure: The author declares no competing financial interests.